How do autoimmune diseases unfold?
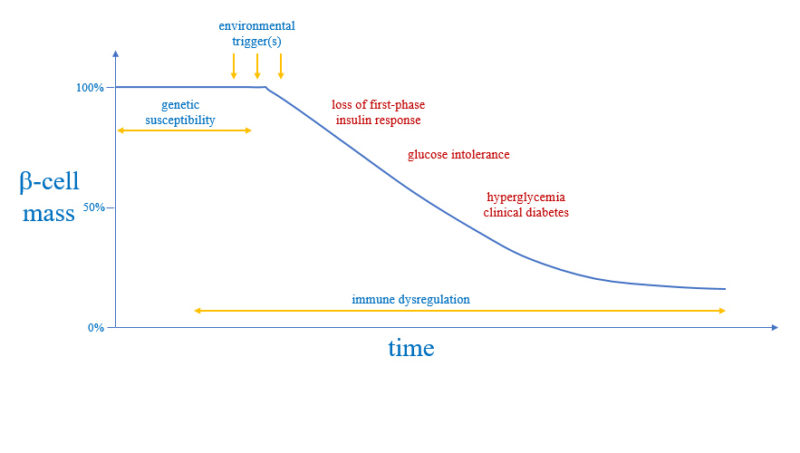
Although we do not know what causes autoimmune diseases, we have learned a great deal about their "pathogenesis" (or "natural history")—how the disease progresses over time and damage ensues.
Three factors are at play in the pathogenesis of autoimmune diseases: genes, immune system, and the environment where the patient lives. The genes confer what is called "predisposition" or genetic susceptibility. The immune system becomes dysregulated and provides the tools for executing the pathological damage. The environment delivers the triggers that may make the autoimmune disease clinically apparent.
We have also learned that autoimmune diseases are chronic conditions: they require a long time (years) before they become clinically evident and diagnosis (so, they have long latent phase), and then last for decades (often a life time) once diagnosed.
Genes
An undeniable role for the genes
Autoimmune diseases tend to occur in the same family (the so-called "familial aggregation)". Similarly, the concordance rate of a given autoimmune disease in identical twins (typically between 25% and 50%) is about 10 times higher than that in fraternal twins (typically between 2% and 8%). These observations indicate that autoimmune diseases are strongly influenced by the patient's genes.
Is there an "autoimmune disease gene"?
Decades of effort have been devoted to study the genetics of many autoimmune diseases, with the main goal of identifying the autoimmune gene or genes. Results have shown that there isn’t a single autoimmune gene.
Autoimmune diseases, for the vast majority of cases, do not fit any simple pattern of inheritance. On the contrary, they are thus considered polygenic (multifactorial) diseases.
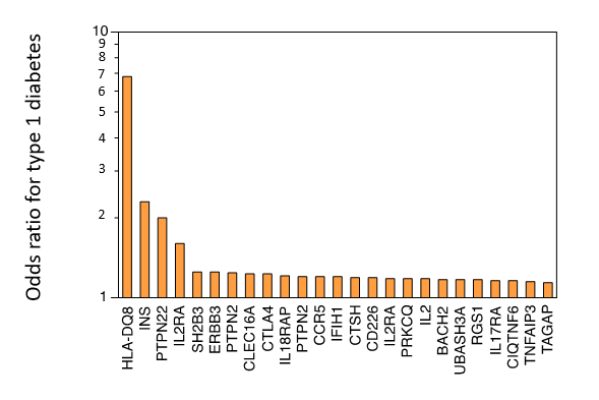
Different Genes, Different Odds
Numerous genes have been shown to increase the risk of developing autoimmune diseases. The individual impact of each of these genes, however, is very small. In other words, each gene confers only a small increase in susceptibility, measured in the order of percent change (an odds ratio, OR, comprised between values of 1 and 2), rather than in fold change (an OR ratio greater than 2).
Among the constellation of genes that have been associated with increased susceptibility to autoimmunity, the brightest star is unquestionably the MHC locus (in humans called HLA locus). For most autoimmune diseases where genetic studies have been performed, HLA has emerged as the locus with the strongest association: for example, the DQ8 allele in type 1 diabetes (OR around 7), DR3 in Sjögren syndrome (OR around 9) and Graves disease (OR around 3), DR2 in ulcerative colitis (OR around 4), etc.
As one moves away from the HLA locus, the strength of the association markedly decreases (odds ratios smaller than 2). The graph adapted from Concannon P et al (N Eng J Med, 2009) shows some of the 50 or so genes that have been associated with type 1 diabetes and their strength of association.
Autoimmune Genes are Scattered throughout the Genome
Autoimmune genes are found in many chromosomes. For example, in systemic lupus erythematosus, over 80 genes have been identified in each of our 22 plus XY chromosomes, as nicely illustrated in this drawing (from Chen L et al, Curr Opin Rheumatol, 2017).
Since autoimmune disease genes are many and individually have low impact, they have been particularly challenging to study.
Rarely, autoimmune diseases are caused by a single gene defect
In a handful of cases, autoimmune diseases are monogenic, that is arise from defects in a single gene. Although rare, these monogenic autoimmune diseases have yielded fantastic insights into autoimmunity because disease manifestations are easier to understand when deriving from a single defect.
Five examples of autoimmune diseases caused by a single gene defect are shown in the table below.
| APS-1 | ALPS | IPEX syndrome | Immunodeficiency 41 with autoimmunity | CVID 8 with autoimmunity | |
|---|---|---|---|---|---|
| Affected gene | AIRE | Fas (type 1a) FasL (type 1b) caspase 10 (type 2a) caspase 8 (type 2b) PRKCD (type 3) NRAS (type 4) CTLA-4 (type 5) | FOXP3 | IL2 receptor A | LRBA |
| Mode of inheritance | Autosomal recessive | Autosomal recessive | X-linked | Autosomal recessive | Autosomal recessive |
| Median age of onset | 3 years | variable | 2 weeks | 1 month | 2 years |
| Defining clinical features | At least 2 of these major features: muco-cutaneous candidiasis, Addison disease, hypoparathyroidism | Lymphadenopathy, splenomegaly, autoimmune cytopenias, other types of autoimmune diseases | Dermatitis colitis, hemolytic anemia, thyroiditis, type 1 diabetes, exaggerated response to viral infections | Recurrent viral, fungal, and bacterial infections, lymphadenopathy, autoimmune enteropathy, dermatitis | Recurrent respiratory infections, hemolytic anemia, idiopathic thrombocytopenic purpura, inflammatory bowel disease |
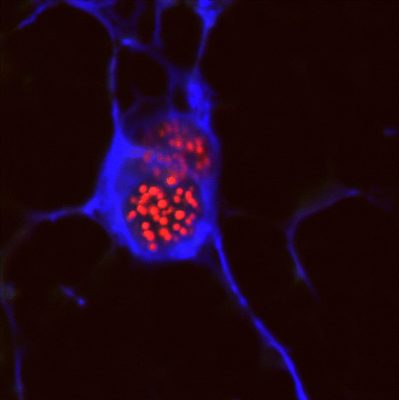
APS-1: The Most Studied Autoimmune Disease Caused by a Single Gene Defect
APS-1 is characterized by at least two of three clinical features: muco-cutaneous candidiasis, Addison disease, and hypoparathyroidism. Patients also have many additional organ-specific autoimmune diseases (such as thyroiditis, type 1 diabetes, vitiligo, autoimmune gastritis, premature ovarian failure, testicular failure, etc). There is great variability in the types of autoimmune diseases patients with APS-1 develop, as well as in the time these diseases develop, even when the AIRE mutation is the same and patients belong to the same family.
In APS-1 the defective gene is called AIRE (for autoimmune regulator).
AIRE plays a key role in establishing tolerance to self-antigens. AIRE is expressed in the nucleus of medullary epithelial cells in the thymus (see photo of a mTEC cell with AIRE protein in red) where it orchestrates the expression of peripheral self-antigens, such as insulin of the pancreatic beta cells or the acetylcholine receptor of the neuro-muscular junctions. T lymphocytes that mature in the thymus interact with mTEC cells: if by chance T cells have an antigen receptor that binds well to these peripheral self-antigens expressed by mTEC cells they are destroyed, and therefore do not exit the thymus to become part of the T-cell repertoire we have in the blood and secondary lymphoid organs (spleen and lymph nodes). If AIRE is mutated, however, a number of peripheral self-antigens are not presented anymore on mTEC cells, and thus the T cells that recognize them are not deleted in the thymus but rather escape into the periphery where they are available to cause autoimmune diseases.
Immune System
Patients with autoimmune diseases have multiple defects in regulatory mechanisms that normally prevent autoreactive lymphocytes to develop or control them if they do appear in the periphery.
Cellular Immunity
Numerous defects in cellular immunity have been demonstrated in animal models of autoimmune diseases or other experimental settings. Examples include decreased number or function of regulatory T cells, resistance of effector T cells to immune regulation, defective function of antigen-presenting cells (such as dendritic cells and macrophages). In the clinical setting, however, these studies of cellular immunity are still not popular because of high costs, technical difficulties (such as limited access to the organ targeted by autoimmunity), and overall feasibility.
Autoantibodies
Autoantibodies on the other hand (and the B lymphocytes that produce them) remain the main clinical indicator of an immunological dysfunction in patients with autoimmune diseases. For a long period of time, autoantibodies were considered a mere biomarker of disease and received limited attention in regards to their pathogenic role.
Recent years, however, have seen a resurgence in the interest on autoantibodies, both in terms of their diagnostic and predictive value. The presence of autoantibodies has taught us that immunological abnormalities are present years before the clinical diagnosis of an autoimmune disease.
For example, antibodies to glutamic acid decarboxylase-65, protein tyrosine phosphatase receptor type N, and insulin predict by several years the development of type 1 diabetes in asymptomatic first-degree relatives. Similarly, antibodies to Ro and La are present at least 5 years before the clinical diagnosis of systemic lupus erythematosus. And antibodies to thyroperoxidase precede by at least 7 years the clinical diagnosis of Hashimoto thyroiditis.
These critical observations were made using the Department of Defense Serum Repository. Since 1985, this repository stores sera of active-duty military personnel as they are collected (every two years of service, and before and after each deployment). When soldiers are then diagnosed with an autoimmune disease, it is thus possible to check what was their autoantibodies status in earlier years, when they were asymptomatic and healthy.
Environment
A role for the environment
The incidence of autoimmune diseases has increased remarkably since they were first recognized as distinct medical entities in the 1950s. Part of the increase is undoubtedly secondary to a greater awareness in the medical community about these conditions. But the increase is too large to be explainable only by better diagnostics. Considering that human genes do not change in 50 years, that the concordance rate of a given autoimmune disease among monozygotic twins is not 100%, and that there is variation in prevalence of the same disease according to geographical locations, investigators have long looked at the environment to identify causes for autoimmunity.
A difficult topic to study
Of the three factors that contribute to the pathogenesis of autoimmune diseases (genes, immune system, and environment), environment is the one where firm, scientifically sound conclusions have been the most limited. Part of the difficulty arises from the fact that each individual autoimmune disease is not very common in the general population. This makes it very challenging to identify environmental factors associated with that disease, also because these factors are expected to confer only a small increase in risk. In addition, autoimmune diseases themselves can be difficult to categorize and define, have variable age at onset, and poorly predictable age at onset. These difficulties have meant that pretty much every possible environmental factor, from poor air quality and pollution to the ever-elusive local viral infection, has been associated with one autoimmune disease or another.
Some environmental factors that have been implicated with autoimmunity are:
- infections (usually viral)
- dietary components (feeding infants with cow’s milk instead of mother’s milk, gluten, lack of vitamin D, increased ingestion of iodine)
- pollutants (chemicals like polychlorinated biphenyls)
- drugs (such as streptozotocin, a form of chemotherapy)
- stress