Molecular Genetics Laboratory of Female Reproductive Cancer
The long-term objectives of our research team are to:
- understand the molecular etiology of female reproductive cancers
- elucidate the mechanics of chemoresistance in ovarian cancer
- translate basic research into potential clinical promise
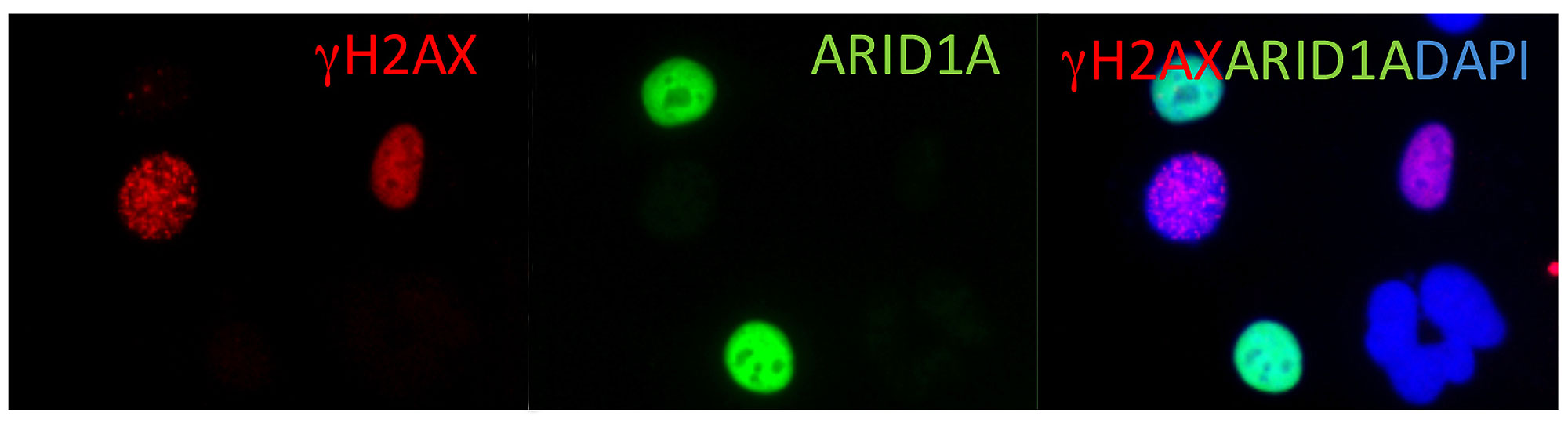
We use ovarian carcinoma as a primary disease model because it is one of the most aggressive neoplastic diseases for women. Our research team aims to understand the molecular basis behind how normal cells are transformed, selected, and evolve to become invasive carcinoma. Genome-wide analyses at Hopkins have identified molecular genetic alterations in different types of ovarian cancers including NOTCH3, ARID1A, PIK3CA, PPP2R1A, among several others. We are currently focusing on revealing the tumor-promoting functions of NOTCH3 signaling and tumor-suppressor functions of the ARID1A pathway. In addition, we are characterizing metabolic reprograming during the development of platinum drug resistance. The ultimate goal is to translate our research findings into new preventive and therapeutic strategies. We are in collaboration with a team of dedicated clinicians and scientists across multiple disciplines to achieve this goal.
What we observe is not nature itself, but nature exposed to our method of questioning.
— Werner Heisenberg, Physics and Philosophy, 1958

Tian-Li Wang, PhD
Director
Biography
[email protected]
Professor; Director of the Molecular Genetics Laboratory of Female Reproductive Cancer
Departments of Pathology, Oncology, and Gynecology & Obstetrics
– Faculty in Pathobiology Graduate Program
– Faculty in Cellular and Molecular Medicine (CMM) Graduate Program
Trainings:
– Johns Hopkins Medical Institutions
– Johns Hopkins University School of Medicine, PhD (study with Bill Guggino and Garry Cutting)
– University of Pennsylvania, School of Medicine, Post-doc fellow – Neuroscience (study with Peter Sterling and Noga Vardi)
– Howard Hughes Medical Institutions, Associate – Cancer Genetics (study with Bert Vogelstein)
Patents:
– Digital Karyotyping US7704687B2
– Small molecule compounds targeting pbx1 transcriptional complex WO2016172437A3
Faculty Collaborator

Ie-Ming Shih, MD, PhD
Co-Director, Breast & Ovarian Cancer Program
Sidney Kimmel Comprehensive Cancer Center
Director, Telinde GYN Pathology Research Program
[email protected]
Richard W. TeLinde Distinguished Professor
Department of Gynecology & Obstetrics
Departments of Pathology and Oncology
Faculty in Pathobiology Graduate Program
Johns Hopkins University School of Medicine
Taipei Medical University, MD
University of Pennsylvania, PhD
Johns Hopkins University, Residency (Pathology)
Johns Hopkins University, Fellowships (Gynecologic Pathology and Cancer Genetics)
Faculty Collaborator

Stephanie L. Gaillard, MD, PhD
Director of Gynecologic Cancer Trials
Associate Professor of Oncology
Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University
[email protected]
Duke University, MD, PhD
Johns Hopkins University, Internal Medicine and Oncology, Resident and Fellowship
Johns Hopkins University, Molecular Genetic Lab of Female Reproductive Cancer, Research Fellow
Additional Collaborators
- Dr. Denis Wirtz
Theophilus Halley Smoot Professor, Department of Chemical and Biomolecular Engineering, Johns Hopkins University - Dr. Peng Huang
Associate Professor, Department of Oncology, Division of Biostatistics and Bioinformatics, Johns Hopkins University - Dr. Thomas Pisanic and Dr. Jeff Wang
Department of Mechanical Engineering, Johns Hopkins University
Acknowledgements
We gratefully acknowledge funding support from the following sources who have made our work possible.
Private Foundations
- Ovarian Cancer Research Fund Alliance (OCFRA)
- The Honorable Tina Brozman Foundation – Tina’s Wish
- Colleen’s Dream Foundation
- Team Katie Oppo – Ovarian Cancer Research Fund
- Endometriosis Foundation of America
- Ephraim & Wilma Shaw Roseman Foundation
- American Cancer Society
- Gray Foundation – for BRCA research
Government funding
- National Institutes of Health/National Cancer Institute
- Department of Defense – Ovarian Cancer Research Program