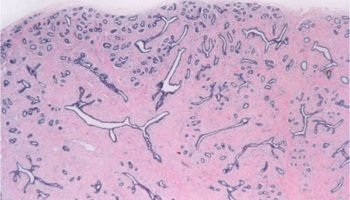
A benign change of the breast where the stroma becomes dense, similar to scar tissue, which distorts the surrounding tissue.
A change in a cell’s DNA. Some mutations lead to a favorable change in a gene or a protein’s function, an unfavorable change, a loss of function, or no change at all (see also genetic mutation).
An imaging technique that uses beams of radiation (X-rays) to take an image of the body. A mammogram is a specialized form of an X-ray image.
Benign hyperplasia (growth) of the breast epithelial cells lining the ducts and lobules.
A multidisciplinary meeting of the physicians and caretakers involved in cancer care, including pathologists, surgical oncologists, medical oncologists, radiologists, nurses and genetic counselors to discuss the treatment plans for individual patients.
A mass or a lump. A tumor mass can be nonneoplastic and be due to something like swelling or inflammation. A tumor mass can also be neoplastic, and includes both benign and malignant tumors.
The process by which the body reads the code in RNA to make proteins.
The process by which DNA is copied to make RNA.
An imaging technique that uses digital 2D mammogram images to build a 3D image of the breast.
The unit in the breast comprised of the lobules and their associated terminal duct. The breast contains innumerable terminal duct lobular units. All breast carcinomas arise from the epithelial cells in the terminal duct lobular unit.
A type of treatment that specifically targets a single molecule or pathway involved in cancer cell growth and progression. Examples in breast cancer care include the drugs that target HER-2, such as trastuzumab and pertuzumab.
A treatment that can reach cancer cells that have potentially spread throughout the body. Examples include chemotherapy, hormone therapy, and targeted therapy. Systemic therapies can have side effects due to effects on normal body cells, such as hair loss or gastrointestinal distress.
A disease that widely affects the entire body.
Any change noted by a patient that could be caused by a disease.
A physician who specializes in surgical treatment (removal) of cancer.
The connective tissue of the breast, that is, the fibrous tissue in which the epithelial elements are located.
A core needle biopsy that is performed with the use of a mammogram to identify the lesion and guide the biopsy needle.
The term for the usual treatment given for a particular disease, which is based on past research and experience proving the treatment’s efficacy and safety.
A core needle biopsy that is done with the use of an ultrasound (sonogram) to localized the mass and guide the needle into the correct location.
A measure of how much a cancer has grown and/or spread in the body (i.e., how advanced a cancer is). The most common staging system is the TNM system, which stands for Tumor, lymph Nodes, and Metastasis. Stage ranges from 0 to 4, with stage 0 being pre-invasive disease (such as DCIS), and stage 4 being metastatic disease. The stage is often written using Roman numerals: stage 0, stage I, stage II, stage III and stage IV. Stage is a prognostic factor, such that a high stage is associated with a poorer prognosis or outcome.
A gene mutation that occurs spontaneously in the body tissues or in the cancer cells that cannot be passed on to offspring (i.e., it cannot be inherited).
The process of surgically removing the sentinel lymph nodes to evaluate them for cancer.
The process of having a second set of doctors look at your unique medical situation to provide a second opinion on the diagnosis and/or treatment plan.
The first lymph node in the chain of regional nodes that drains the lymph fluid from the breast. The sentinel lymph node is most commonly located in the axilla (underarm) and is typically the first lymph node that will be involved by cancer when the cancer starts to metastasize.
A term used to describe a test used to look for a disease before it has caused symptoms. Mammograms are the primary screening test used to look for breast cancer in women.
A benign proliferation of breast glands.
A cancer that arises from the connective tissue of the body. Examples include angiosarcoma (arising from blood vessels) and leiomyosarcoma (arising from smooth muscle cells).
RNA molecules are a copy of the genetic information encoded in DNA, and the RNA copy is then used to create proteins.
A term used to describe the balance between the risk (such as side effects) and benefit of a therapy, procedure, or other course of action.
Anything that increases the risk of developing a disease. For breast cancer, these include family history and age.
The chance or probability of developing a disease in a given period of time.
A research study in which patient records and files are reviewed to look for results (outcomes) that already occurred in the past.
Resident physicians are physicians who have finished medical school and are now studying a specific area in depth, such as pathology, internal medicine, surgery, pediatrics, radiology, and more.
A reduction is size.
Lymph nodes that drain (collect) the lymph fluid from a particular part of the body. The main regional nodes of the breast are in the axilla (underarm), but also include those the infraclavicular (under the collarbone), supraclavicular (above the collarbone), and internal mammary (beneath the pectoralis muscle) lymph node chains.
When a cancer returns after previously having been eliminated. This can be a local recurrence in the area of the breast where the cancer was first detected, or a distant recurrence when the cancer metastases to a new organ.
A type of research study in which patients are randomly assigned into treatment groups, either to receive an experimental treatment (“intervention group”) or standard treatment (“control group”).
A physician who specializes in the use of imaging techniques, such as X-rays, mammograms, CT scans, and MRIs. This can include reviewing scans to detect a physical abnormality or mass, or for placing a needle in an exact location in order to perform a core biopsy.
A treatment for some forms of cancer that uses high energy radiation to damage the DNA of the cells. This is a form of local therapy to the breast or other area such as the bones.
A physician who specializes in using targeted radiation therapy to kill tumor cells in a specific area.
Similar to a complex sclerosing lesion, a radial scar is a benign lesion in the breast which contains scar-like changes in the stroma and angulated glands.
A type of biopsy that samples the skin and immediately underlying tissue by removing a small circular/columnar piece of tissue.
The surgical removal of breast tissue to prevent cancer from developing. One scenario in which this might be performed is in patients who have a high risk of developing breast cancer in the future, such as those with known germline mutations in BRCA1 or BRCA2.
A term used describe treatments that are done before a disease occurs to prevent the disease from happening.
A research study that is conducted using new patients and following their course to observe the outcome
A measure of how rapidly a tumor is growing by assessing how many cells are dividing. The measure ranges from 0% (no cells dividing) to 100% (all cells dividing). (See also Ki67.)
A term used to describe continued growth of a cancer.
A test result that can be used to help predict a patient’s prognosis. For instance, the expression of the estrogen receptor (ER) and progesterone receptor (PR) are favorable prognostic features.
A term used to describe the expected outcome of a cancer or disease (i.e., favorable or unfavorable).
The protein responsible for binding to and detecting progesterone in the body; the receptor is located in the nucleus of many cell types, including the breast epithelial cells. Some cancers express the progesterone receptor and are termed “hormone receptor positive” cancers.
A test result that can be used to predict if a therapy will be effective. Predictive biomarkers help guide treatment choice. For instance, HER-2 positivity is a predictive biomarker that predicts the tumor will respond to HER-2-targeted therapy.
A sex hormone made by the body that is part of the estrogen signaling pathway
Before menopause.
After menopause.
A rare type of fibroepithelial lesion of the breast that contains neoplastic stromal cells and associated benign epithelium. Phyllodes tumors differ from fibroadenomas because they are more cellular and have a unique “leaf-like” pattern of growth. Phyllodes tumors can be benign, borderline (i.e., in between benign and malignant), or malignant and all require surgical excision.
A term that describes variation in size and shape of a cell’s nucleus.
A physician who specializes in breast reconstructive techniques to reconstruct the shape of a breast after mastectomy.
A type of imaging study that uses a radioactive element attached to a
sugar molecule to detect parts of the body with rapidly growing cells (which
consume more sugar), such as cancer cells.
Near and around the time of menopause.
A method of processing tissue to evaluate it under the microscope; the tissue is formalin fixed and paraffin embedded so that it can be thinly sliced and made into slides to review under a microscope.
A physician who specializes in the diagnosis of disease; pathologists use a microscope to examine the cells from tissue to determine if the tissue is normal or cancer.
A description for how a cancer has responded to therapy, as seen under the microscope.
The tissue of an organ.
Treatments given to relieve pain and symptoms rather than to cure the disease.
The presence of carcinoma in situ cells within skin (epidermis) of the nipple
and areola.
A surgical procedure where the ovary is removed.
The part of a cell that contains the cell’s genetic material, DNA.
Hidden, or not known.
The size ratio of the nucleus to the cytoplasm. In many cancers, the nuclear becomes markedly and abnormally enlarged, leading to the abnormal feature of a “high N:C ratio.”
A histologic measure of how closely a cancer cell nucleus resembles that of a normal cell, or a measure of how abnormal a cancer nuclear is. It is generally graded as 1 (resembles normal), 2 (moderately abnormal), and 3 (markedly abnormal).
Relating to the nucleus of a cell.
The location where the large milk ducts meet to release milk protein during lactation.
An abnormal growth of cells that are clonal, that is, they arose from each other and share genetic material. Neoplasms can be benign or malignant.
Therapy that is given to the patient before surgery to attempt to shrink the tumor size. Neoadjuvant therapy is typically chemotherapy or targeted therapy, but can also include hormonal therapy or radiation therapy.
The cell that makes the breast’s stroma or fibrous tissue. Breast myofibroblasts can also respond to changes in estrogen levels in the body.
Placement of a metal wire into a mass by a radiologist to assist the surgical oncologist (surgeon) in locating the mass at the time of surgery.
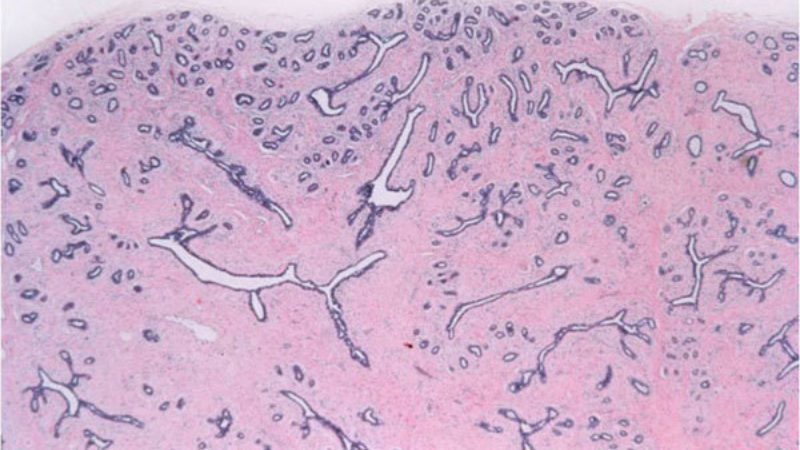
The cell that surrounds normal breast ducts and and lobules; myoepithelial cells have some contractile properties and also produce the normal basement membrane that surrounds the ducts and lobules. Myoepithelial cells are still present around ducts that contain carcinoma in situ, however, invasive carcinomas lack myoepithelial cells.
An approach to patient care that incorporates several disciplines of medicine and allows for communication between physicians and caretakers of different specialties. In breast cancer care, this includes genetic counselors, medical oncologists, nurse navigators, pathologists, radiation oncologists, radiologists, and surgical oncologists. By sitting everyone down at one time, medical providers can better coordinate care, leading to better patient care.
An imaging technique that uses a powerful magnetic field and radio waves to take pictures of tissue deep in the body.
Classification of cancer based on its gene expression.
A count of number of dividing cells in a sample. Mitotic counts are generally measured by number of mitotic cells per 10 high power fields (HPF). The more mitotic cells present, the faster the cells are growing.
The process by which a cell divides into two cells. Under the microscope, dividing cells can be identified by their exposed chromosomes (DNA).
A device used by pathologists to examine tissue on slides; the microscope magnifies the tissue so that pathologist can examine the individual cells and make a diagnosis.
A benign growth location within the terminal duct lobular units. It does not require surgical excision.
Small clusters of calcium that are visible on mammogram and can be associated with either benign processes (such as fibrocystic changes) or atypical processes (such as DCIS or invasive cancer).
Breast cancer which is spread beyond the breast and is growing in a distant organ such as the bones, liver, lung or brain.
The spread of and presence of cancer cells that have spread to other organs in the body outside of the primary site.
A natural process during which a women ceases to have a menstrual period; this typically occurs in the late 40s-50s. The ovaries no longer ovulate (i.e., no longer produce eggs) and no longer produce estrogen hormone.
A doctor specialized in the treatment of cancer using hormonal therapy, chemotherapy, and targeted therapy.
A procedure whereby a surgeon removes the breast.
Inflammation of the breast ducts.
A lump or swelling. A mass can be due to excess fluid or an abnormal growth of cells; the growth of cells can be benign or malignant.
The ‘edge’ of specimen containing a rim of normal-appearing tissue around the tumor. Pathologists evaluate the margin tissue under the microscope to see if the tumor has been entirely removed. “Negative” or clean margins means that all of the tumor was removed. “Positive” or involved margins means that the tumor was not entirely removed and additional surgery may be necessary
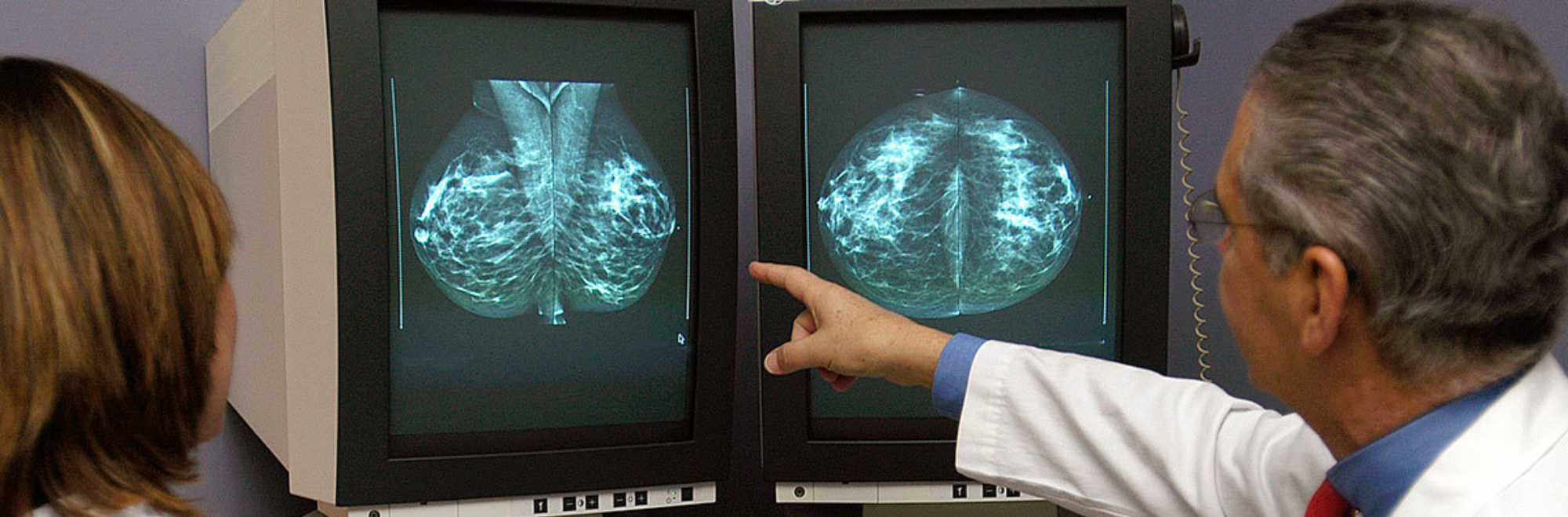
A radiology test that takes X-ray images of the breast used to look for abnormalities including masses or abnormal patterns of microcalcifications.
The ducts and lobules in the breast that produce milk protein; ducts meet together at the nipple to release milk onto the skin surface.
Relating to the breast or breast tissue.
Cancer cells with the ability to invade surrounding tissue and with the potential to metastasize (spread) to lymph nodes and distant organs.
The presence of cancer cells spreading into lymphatic channels.
A type of white blood cell belonging to the immune system. Lymphocytes have many functions, including fighting viruses and cancer.
The small channels that carry the lymph fluid throughout the body and drain through lymph nodes.
Swelling of the body due to a build-up of the lymph fluid; this can occur in the hands and arms after the removal of lymph nodes during surgery.
Small organs comprised of groups of lymphocytes (immune cells) that filter the lymph fluid that flows through the lymphovascular or lymphatic channels through the body. Cancers often first use the lymphatic channels to spread through the body. Breast cancers typically first spread to the lymph nodes in the axilla (underarm) before spreading elsewhere in the body.
A surgical procedure where a limited amount of tissue is removed from the breast, to include both the mass and immediately surrounding tissue.
A mass.
A group of cancers defined by the expression of certain genes; most luminal breast cancers are positive for the estrogen receptor (ER).
The epithelial cells that line the breast ducts.
Cancer that has not yet spread to nearby tissues (by direct invasion) or to distant organs (by metastasis).
See breast lobule.
A treatment that targets just the anatomic area of tumor; for instance, surgery and radiation to the breast are local therapies.
An abnormal proliferation of cells within the breast ducts and lobules; these cells lack E-cadherin expression and may be precursor cells to invasive lobular carcinoma. The presence of LCIS increases a patient’s risk of subsequently developing cancer, in both breasts.
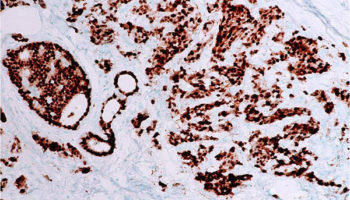
An immunostain that marks a gene that is involved in cell proliferation or growth. The degree of Ki67 labeling in a cancer cell correlates to how quickly the tumor is growing and how aggressive it is. The measure ranges from 0% (no cells dividing) to 100% (all cells dividing). See also proliferation index.
A pattern of growth where the cancer cells grow into (invade) the surrounding tissues (see also infiltrating).
Something that occurs during an operation. For instance, a frozen section is done intraoperatively.
A lymph node located within the breast
A benign papillary growth of cells located entirely within the ducts of the breast. This may cause nipple discharge.
"Within the duct." This refers to something that is entirely located within a duct and does not grow beyond the borders of the duct wall.
A rare and aggressive type of breast cancer where the cancer cells infiltrate the lymphovascular spaces within the skin dermis, causing changes in the overlying breast skin. The skin often looks swollen, thickened, dimpled, and red.
The result of the presence of immune cells (“inflammatory” cells) to a part of the body. Areas of the body that are inflamed often look swollen and red.
A pattern of growth where the cancer cells grow into (invade) the surrounding tissues (see also invasive).
A type of treatment that uses the immune system to fight cancer; these therapies target proteins expressed by immune cells or on the cancer cell.
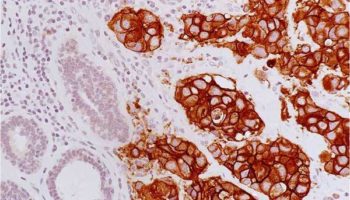
A type of laboratory test that can detect the proteins expressed by a cell. The test uses special antibodies (“immunostains”) that each binds to a particular protein in question; the immunostain will change the color of the tissue to show whether a protein is present. Examples include immunohistochemistry to look for HER-2 overexpression, as well as the expression of the estrogen receptor (ER) and progesterone receptor (PR) in breast cancer cells.
The body’s natural defense against infection with microorganisms such as bacteria and viruses. The immune cells are constantly on the lookout for cells that look “foreign.” In addition to microorganism, immune cells can also recognize cancer cells as “foreign,” since the cancer cells may express abnormal proteins. In this way, the immune system can sometimes be a part of the body’s attack against cancer.
Increased numbers of cells. This can be benign (see usual duct hyperplasia) or atypical (see atypical ductal hyperplasia).
A receptor protein that binds hormones within a cell in order to affect changes within the cell. Tumors with high numbers of hormone receptors need hormones to grow.
A substance released into the blood that influence how other tissue behave and grow. Examples include estrogen, progesterone, and androgen.
A form of systemic treatment that blocks hormones from getting to the cancers that have hormone receptors. One example is tamoxifen.
Relating to appearance of cells and tissues under the microscope.
A molecular gene signature associated with HER-2 gene amplification.
The area of tissue that is seen at a microscope’s highest magnification (i.e., the most “zoomed in”).
A protein called a tyrosine kinase that is expressed on the surface of all cells and helps signal for the cells to grow. Some cancers overexpress HER-2/neu on their surface, that is, they have more copies of the gene and thus protein than normal; these are called “HER-2 positive” tumors. This HER-2 overexpression allows the cells to grow abnormally. There are several types of drugs that directly target the HER-2 protein on cancer cells; one example is trastuzumab.
A type of dye that is applied to tissue sections so that the cells absorb the color and can be seen with the eye when looking under the microscope. This dye turns the nuclei blue and the cytoplasm pink.
A small, thin rectangular piece of glass where tissue slices from a biopsy or a surgical specimen are placed and stained with dye so that the tissue can be evaluated under a microscope.
A histologic description of how closely the cancer cells resemble their normal cell of origin. In the breast, the overall grade score is calculated by looking at the mitotic rate, the nuclear grade or atypia, and the degree of gland formation. The final grade will be either grade 1, 2 or 3. In general, a higher tumor grade is associated with more aggressive behavior.
Tissues in the body that make protein secretions; glands are shaped like small round structures. In the breast, the ducts and lobules are the glandular tissue that produces milk protein. Cancers that arise from glands are called “adenocarcinomas” (see also mammary gland).
A mutation in DNA that is present at birth and that can be transferred from parent to child.
A change in a cell’s DNA. Some mutations lead to a favorable change in gene or protein’s function, an unfavorable change, a loss of function, or no change at all (see also mutation).
A test of a patient’s DNA to look for specific gene mutations or other abnormalities that might cause cancer or other conditions.
A member of the healthcare team specialized in diagnosing and interpreting genetic test results. A geneticist might be consulted to help understand germline or somatic mutations.
A meeting between a patient and a medical geneticist or counselor to discuss the potential impact of a genetic test result on the health of a patient and for their family.
A laboratory test that analyzes the expression of multiple genes to characterize what proteins tumor cells are creating.
A single sequence of DNA that codes for a protein.
A method that pathologists can perform intra-operatively (i.e., while a surgery is underway) to quickly freeze a piece of tissue from the patient in order to take thin slices and make a slide to evaluate “in real time” while the surgery is still ongoing. This is sometimes performed on breast sentinel lymph nodes to tell the surgeon whether there are metastatic cancer cells there. The results are only preliminary, however, and must be confirmed with review of the final FFPE sections.
A term used to describe how fresh tissue samples are processed and stored so that slides of the tissue can be made and examined by a pathologist. The fresh tissues are “fixed” in a preservative called formalin, so that the tissues will not degrade or decompose. They are then “embedded” into paraffin wax, which means they are placed into a little block or wax similar to candle wax so that they can be easily sliced into thin slices and placed on a glass slide for a pathologist to review.
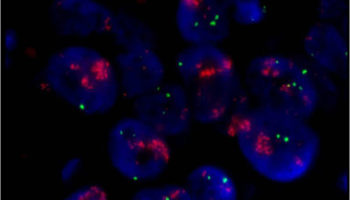
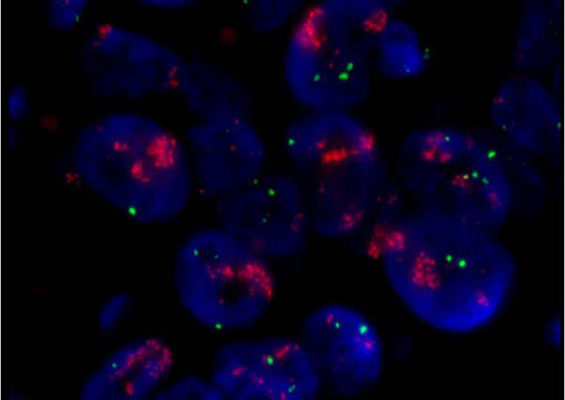
A laboratory test that can be used to look for HER-2/neu gene amplification in breast cancers. This test uses a probe molecule that has an attached fluorescent tag; when the HER-2/neu gene is present, the probe binds to the gene and shines brightly. The number of genes can be counted using a special fluorescent light microscope.
A type of biopsy in which a hollow needle is used to aspirate (suck out) small clusters and individual cells from a mass.
A category of tumors that have contain proliferations of both stromal and epithelial cells. This category includes fibroadenoma and phyllodes tumor.
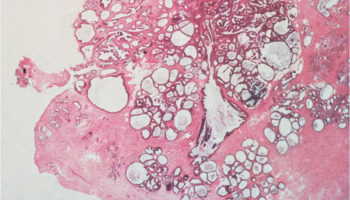
A normal, benign process that occurs in the breast in which the tissue develops small or large cysts, benign epithelial proliferation, and increased fibrosis. This can cause the breast to feel lumpy or bump. These changes can be painful and typically change during the course of the menstrual cycle.
A benign breast lesion containing both the stromal and epithelial cells. Fibroadenomas are the most common type of fibroepithelial lesion of the breast, and they are most common in young adult women. Fibroadenomas do not require surgical excision, but they may be excised if they are growing or symptomatic such as causing pain.
A process that results when fat cells die; these fat cells make up the adipose tissue of the breast, and they can undergo fat necrosis due to injury or surgery. Fat necrosis can form a palpable mass or create a mass seen on imaging, leading to the need for a core needle biopsy. However, fat necrosis is a benign finding, and it will resolve spontaneously over time.
The medical history of all of the biological (blood-related) members of a family; this family medical history can show patterns of shared diseases. Because you share genes with your family members, a “positive family history” of certain diseases may be considered a risk factor for an individual to develop the disease.
A positive result for a test that should actually be negative (i.e., an incorrect test result that states a person is positive for disease, which the person is actually disease-free).
A negative result for a test that should actually be positive (i.e., an incorrect test result that states a person is disease-free, which the person actually has the disease).
A surgical procedure to remove the abnormal area of the breast to make a definite diagnosis. Excisional biopsies are typically performed either when a core needle biopsy is not possible (i.e., the abnormal area is too deep in the breast tissue to be reached by the core needle), or when a core biopsy has given a diagnosis of atypia and additional tissue is needed to make a definite diagnosis of cancer or benign.
The protein responsible for binding to and detecting estrogen in the body; the receptor is located in the nucleus of many cell types, including the breast epithelial cells. Cancers that express the estrogen receptor are termed “hormone receptor positive” cancers.
The major female sex hormone, responsible for many physiologic functions in the body. Estrogen is made by the ovaries and adrenal gland. Estrogen causes the normal growth of many cell types within the breast. Some cancers require estrogen to grow.
A type of cell in the body that makes up many different tissue types, including the ducts and lobules of the breast. These specialized epithelial cells are called the “ductal” or “luminal” cells of the breast. Epithelial cells in other parts of the body line the body surface (such as the “squamous epithelium” of the skin) and the body cavities. The epithelial cell is the cell or origin of carcinomas.
Swelling of a part of the body from excess fluid (see also lymphedema).
The molecule which contains all of your genes, located within a cell’s nucleus … a long, complex molecule with which your genes are encoded.
The portion of a cell outside the nucleus, but still within the cell membrane.
A benign, fluid-filled space lined with epithelium; cysts can cause a breast to feel lumpy or bumpy.
A type of imaging that uses X-rays to take 3-dimensional images. CT scans are not routinely used to image the breast.
Thin pieces of tissue taken with a thin needle; the tissue is sent to pathologists to evaluate and make a diagnosis. Core needle biopsies of the breast are performed with the use of radiographic imaging done at the same time to guide the needle into the correct location. The imaging used can be a mammogram (“stereotactic core biopsy”), an ultrasound (“sono core biopsy” or “ultrasound-guided core biopsy), or an MRI (MRI-guided core biopsy”).
A consultation in pathology occurs when a specimen is sent to a second (or sometimes third) institution to review the findings. This can occur when other pathologists need assistance with a particularly challenging or rare case, or if a patient or clinician would like a second opinion on a case.
The layer of cells that lines the outside of the body, lines the inside of the body cavities, and lines the outside and inside of body organs. Epithelium is one of four types of tissues in the body; the other three types are connective tissue (like fat and fibrous tissue), muscle tissue, and neural/nervous system tissue. The epithelium lining each of the surfaces in the body has different names; for instance, the epithelium lining the outside of the body is called skin, and the epithelium lining the inside of the chest cavity is called the pleura. The epithelium within the breast consists of a layer of cells that forms the ducts and lobules, which make milk protein during lactation (breast feeding).
A type of benign change of the cells that line the breast that makes them appear taller under the microscope, like a column.
A study organized by a hospital, organization, or other group to systematically and thoroughly investigate a new medication, technique, or other approach to treatment. Clinical trials are extensively monitored to make sure that they are conducted in a safe, ethical, and equitable manner.
A systemic medication used to treat cancer that kills cells that are dividing. Sometimes this results in undesired side effects, such as hair loss, because the chemotherapy drugs also kill normal body cells that are dividing.
The circular area of pigmented (darkly shaded) skin surrounding the nipple on the breast.
Absolute and relative risk are two different ways to measure risk. Relative risk compares risks between groups, while absolute risk is the total chance that something such as a disease will occur in a certain time period.
A type of carcinoma (cancer) that arises from glandular cells.
Adjuvant therapy is any treatment given in addition to surgery. It can include chemotherapy, radiation therapy, or other treatment. This is in contrast to neoadjuvant chemotherapy, which is given before surgery.
A type of DNA mutation in which a single gene is duplicated multiple times to increase its activity.
The process of growing new blood vessels, which cancer cells need in order to grow.
A condition where the breast epithelial cells grow abnormally within the ducts. Atypical ductal hyperplasia is not cancer, but it increases the risk of later developing breast cancer.
Irregular and disorderly growth of the epithelium lining ducts. Unlike usual duct hyperplasia, atypical lobular hyperplasia is considered to be an early stage of cancer. Atypical lobular hyperplasia arises from the lobules of the breast.
A surgical procedure in which the surgeon examines the fat in the axilla (underarm) for lymph nodes, which are subsequently sent to pathology to be examined under a microscope for the presence of cancer.
A portion of breast tissue that extends into the axilla (underarm).
The technical term for the tissue in the underarm area (“armpit”).
Non-cancerous. A benign tumor cannot invade nearby tissues or spread to other parts of the body.
A non-invasive, clonal proliferation of epithelial cells that has does not have the capacity to invade into the normal tissue or to spread through the body. This is sometimes called “pre-cancer.”
A surgical procedure in which a surgeon removes the breast tissue of both breasts.
Any chemical or protein created by the body that can be measured, and can be used to provide useful information such as whether a cancer is growing or shrinking during treatment. Biomarkers can also provide information about the prognosis of a cancer (prognostic biomarker), as well as whether a cancer will respond to certain therapies (predictive biomarker).
A tubular structure that carry blood both to and from various parts of the body. This includes arteries, veins, and capillaries.
A tubular structure that carries milk from the terminal ductal lobular unit to the nipple.
A lobule is a gland that primarily functions to make milk in the breast. A collection of lobules along with a small duct make up the terminal ductal lobular units of the breast.
A specific type of cancer that is classified based on its DNA. These cancers are usually "triple negative", in that they do not express progesterone receptor, estrogen receptor, or HER2/neu.
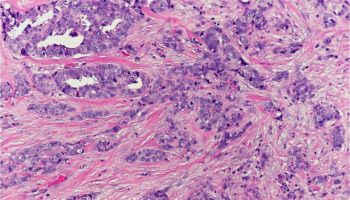
A neoplastic (clonal) growth of cells with the potential to metastasize (spread throughout the body). Cancers can arise from epithelial cells (“carcinomas”), melanocytes (“melanomas"), stromal or connective tissue cells (“sarcomas”), and lymphoid cells (“lymphomas and leukemias”).
Involving “Both Sides”, such as both breasts. This is in contrast to unilateral, which means on one side only.
A type of cancer arising from an epithelial cell.