A deeper look at what we've discovered about bladder cancer
Discoveries
Low-Grade Papillary Urothelial Carcinoma with Degenerative Atypia: A grading Pitfall
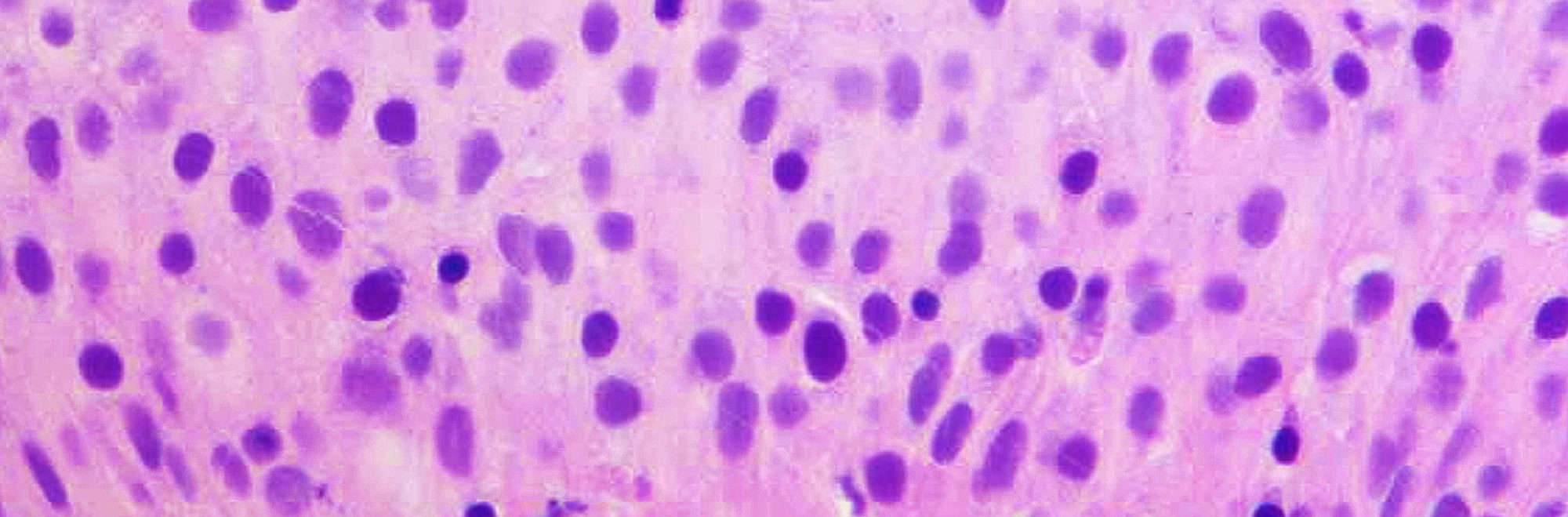
Noninvasive low-grade papillary urothelial carcinoma is a papillary neoplasm with orderly appearance and mild nuclear pleomorphism. Some cases show significant nuclear pleomorphism with degenerative atypia leading to grading difficulties. These are rare tumors characterized scattered cells with nuclei larger than 5 times the size of stromal lymphocytes but displayed smudgy chromatin and occasional multinucleation and intranuclear vacuoles. Next generation sequencing identified the following mutations: HRAS (n=4), FGFR3 (n=3), KRAS (n=3), BRAF (n=1), PDGFRA (n=1), PIK3CA (n=1). Other deleterious mutations were identified but none in genes characteristic of high-grade tumors. The combination of preservation of polarity, low mitotic activity, Ki-67 <5% with the larger atypical nuclei negative for Ki-67, along with nuclear atypia that is degenerative are features used to classify these tumors as low-grade.
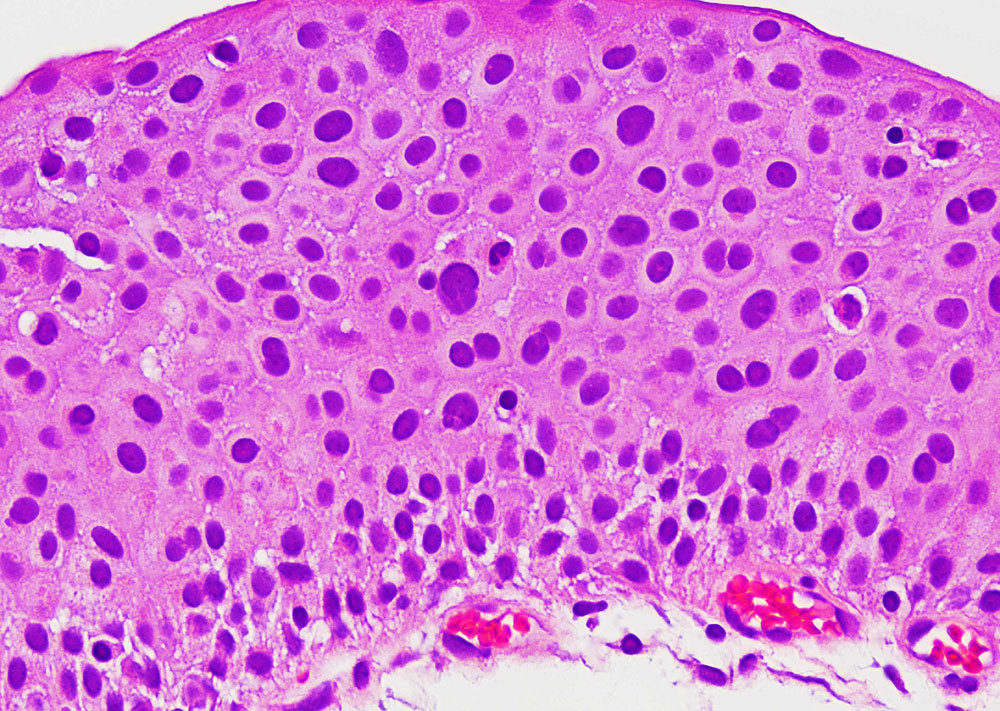
Figure - High power view of thickened urothelium with scattered larger cells with degenerative chromatin
Reference
Matoso A, Parimi V, Epstein JI. Noninvasive Low-Grade Papillary Urothelial Carcinoma with Degenerative Nuclear Atypia: A Grading Pitfall. Human Pathology 2021 Apr 19:S0046-8177(21)00053-8.
Urothelial Carcinoma In Situ of the Bladder: Correlation of CK20 Expression With Adaptive Immune Resistance, Response to BCG Therapy, and Clinical Outcome
Immunohistochemical stains have been suggested to aid in diagnostically challenging cases of urothelial carcinoma in-situ (CIS). Although full thickness immunostaining for CK20 is supportive of CIS, a subset of CIS cases is CK20(-), the clinical significance of which was unknown. This study included 43 patients with primary diagnosis of bladder CIS including 32 with only CIS, 5 with CIS and separate noninvasive high-grade papillary urothelial carcinoma, and 6 with CIS and separate high-grade urothelial carcinoma with lamina propria invasion. Digital morphometric image analysis showed that the average nuclear areas of enlarged nuclei were similar in CK20(+) and CK20(-) CIS (26.9 vs. 24.5 µM2; P=0.31). Average Ki67 index for CK20(+) CIS was higher than CK20(-) CIS (31.1% vs. 18.3%; P=0.03). Patients with CK20(+) CIS [28 (65%)] and patients with CK20(-) CIS [15 (35%)] had the same rates of Bacillus Calmete-Guerin (BCG) failure but patients with CK20(-) CIS had higher stage progression [3 CK20(+) (11%) vs. 6 CK20(-) (40%); P=0.02]. Given recent approval of immune checkpoint inhibitors in patients with CIS refractory to BCG, programmed death ligand-1 expression and colocalization with CD8(+) lymphocytes was investigated as signature of adaptive immune response and was seen in 8 patients regardless of CK20 status and exclusively among patients who failed BCG. Our results confirm that negative CK20 IHC does not exclude CIS and that those patients have similar clinical outcomes as patients with CK20(+) CIS. Programmed death ligand-1 and CD8 colocalization seen among patients who failed BCG therapy is an easy assay to perform to identify patients who could potentially benefit from combined BCG therapy and immune checkpoint inhibition.
Expression of Nectin-4 in Bladder Urothelial Carcinoma, in Morphologic Variants, and Nonurothelial Histotypes
The antibody-drug conjugate enfortumab-vedotin acts by targeting nectin-4, a protein that is nearly ubiquitously expressed in conventional urothelial cancer. However, expression of nectin-4 in morphologic variants of urothelial carcinoma and nonurothelial histotypes was unknown. Immunohistochemistry for nectin-4 using was performed on 169 patients including 83 with nonmuscle invasive bladder cancer and 86 patients with muscle invasive bladder cancer. Staining was scored for intensity (0 to 3) and extent (% positive cells) using the histological score system, where >15 was considered positive. Overall, 72/83 (87%) samples of nonmuscle invasive urothelial carcinoma were positive, including 29/30 (97%) noninvasive papillary urothelial carcinomas, 7/8 (87.5%) carcinomas in situ, 36/45 (80%) papillary urothelial carcinomas invading the lamina propria. Overall, 50/86 muscle invasive tumors were positive, including 15/22 (68.2%) urothelial carcinomas, 7/10 (70%) squamous cell carcinomas, 3/11 (28%) micropapillary tumors, 4/6 (66%) adenocarcinomas, 2/4 (50%) nested carcinomas, 5/8 (63%) plasmacytoid, 1/10 (10%) sarcomatoid carcinomas, and 0/15 (0%) small cell carcinomas. Whole transcriptome RNA sequencing revealed that compared with conventional urothelial carcinomas, most sarcomatoid carcinomas and all but 2 small cell carcinomas expressed very low levels of nectin-4 mRNA but expressed significant levels of either trop2 or ERBB2, which are the molecular targets of 2 other antibody-drug conjugates-sacituzumab gavitecan (trop2) or trastuzumab deruxtecan (ERBB2/HER2). In summary, our study demonstrates that there is heterogeneity of expression of nectin-4 in morphologic variants of urothelial cancer and nonurothelial histotypes, and suggests that testing expression of nectin-4 should be considered in morphologic variants or nonurothelial histotypes found to have lower expression.
Intestinal Metaplasia Without Dysplasia in the Urinary Bladder Reveal Only Rare Mutations Associated With Colorectal Adenocarcinoma
Intestinal metaplasia (IM) is a rare finding in urinary bladder specimens. It is unclear whether IM without dysplasia is a precursor of malignancy in the urinary system. We retrospectively selected cases of IM of bladder, and performed mutation analysis for genes frequently mutated in colon cancer including BRAF, APC, KRAS, MET, NRAS, PIK3CA, CTNNB1, FBXW7, and TP53 using validated clinical tests. One IM case revealed an APC mutation and another showed an NRAS mutation. Clinical follow-up for the IM patients was available with a median follow-up of 70 months. One patient-without any mutation in the genes investigated-developed invasive bladder adenocarcinoma with intestinal differentiation with metastasis to the liver and lung. Neither of the 2 patients harboring mutations developed any malignancy. In conclusion, a minority of cases with IM without dysplasia bear mutations in the genes commonly associated with colonic adenocarcinoma, suggesting a premalignant potential for such lesions possibly following the classic multistep chromosomal instability pathway of carcinogenesis. A larger cohort of patients with longer follow-up is needed to better establish whether close follow-up is warranted for mutation-harboring IM of the bladder.
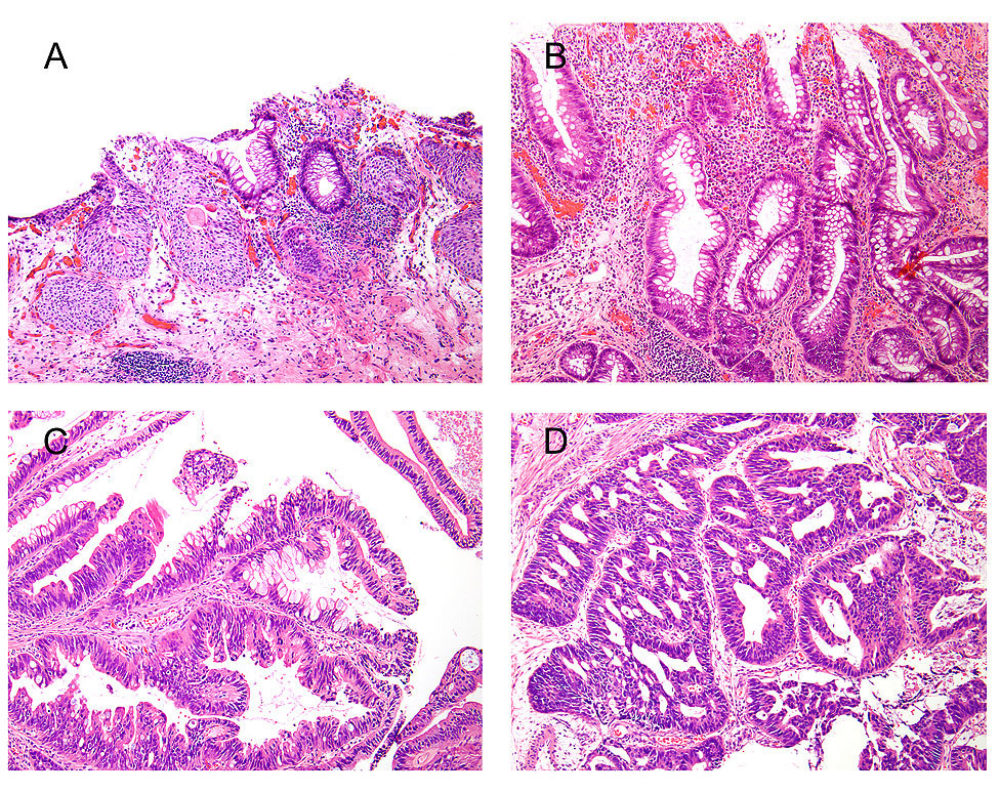
Figure - Spectrum of findings in patients with intestinal metaplasia of the bladder. A, Benign urothelium with cystitis cystica et glandularis and a focus of intestinal metaplasia without dysplasia. B, Focus of intestinal metaplasia without dysplasia and without association with cystitis cystica glandularis. C, Intestinal metaplasia with low-grade dysplasia. D, A case of adenocarcinoma with intestinal differentiation arising in association with intestinal metaplasia.
Reference
Amin A, Murati-Amador B, Lombardo KA, Jackson CL, Grada Z, Palsgrove DN, Matoso A. Analysis of Intestinal Metaplasia Without Dysplasia in the Urinary Bladder Reveal Only Rare Mutations Associated With Colorectal Adenocarcinoma. Appl Immunohistochem Mol Morphol. Nov/Dec 2020;28(10):786-790.
Adaptive immune resistance to BCG
Therapy with Bacillus Calmette-Guerin (BCG) is a
form of immunotherapy where patients develop an immune response that attacks
tumor cells. A proportion of patients are unresponsive to this therapy. We
characterized the immune cell expression among patients with non-muscle
invasive bladder cancer (NMIBC) treated with Bacillus Calmette-Guerin (BCG) and
found no differences in CD4, CD8, or FoxP3 expression between responders and
nonresponders. Baseline PD-L1 expression was observed in 25% to 28% of
nonresponders and 0% to 4% of responders (P < 0.01). PD-L1+ cells in BCG nonresponders colocalized
with CD8+ T cells. In
addition, BCG therapy did not increase PD-L1 gene expression (RNA-seq) or
protein levels. The number of pretreatment CD4+ T cells was very low among PD-L1+ nonresponders (12%) and high among
PD-L1- nonresponders
(50%). One mechanism of BCG
failure may be adaptive immune resistance. Baseline tumor PD-L1 expression
predicts an unfavorable response to BCG and if validated, could be used to
guide therapeutic decisions.
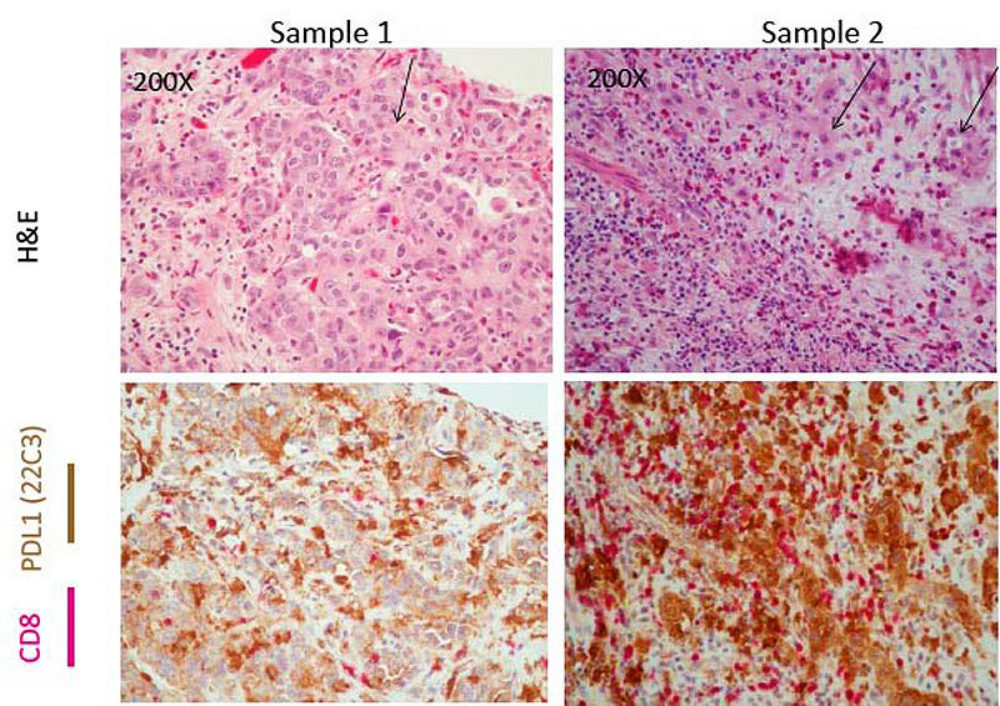
Figure - Colocalization of PDL1 and CD8 among BCG Nonresponders. H&E demonstrates tumor cells in Sample 1 and Sample 2 (demonstrated by the arrow). These tumor areas have evidence of both PDL1 brown staining cells as well as pink CD8+ expressing cells. Taken together, these images suggest colocalization of CD8 and PD-L1 in the tumor microenvironment.
Reference
Kates M, Matoso A*, Choi W, Baras AS, Daniels MJ, Lombardo K, Brant A, Mikkilineni N, McConkey DJ, Kamat AM, Svatek RS, Porten SP, Meeks JJ, Lerner SP, Dinney CP, Black PC, McKiernan JM, Anderson C, Drake CG, Bivalacqua TJ. Adaptive Immune Resistance to Intravesical BCG in Non-Muscle Invasive Bladder Cancer: Implications for Prospective BCG Unresponsive Trials. Clin Cancer Res. 2020 Feb 15;26(4):882-891. *Co-first author
Diagnosis of urothelial carcinoma in situ using blue light cystoscopy
Carcinoma in situ (CIS) is difficult to visualize with white light cystoscopy (WLC), whereas blue light cystoscopy (BLC) using photosensitizing agents improves detection rates. The goal of our study was to assess the sensitivity of BLC and correlate the results with final pathology diagnoses. We also focused on cases that were abnormal under BLC and had a pathology diagnosis that was suspicious, but not diagnostic of CIS. We found that BLC increases detection of CIS that would have been underdiagnosed with the conventional WLC. The lesions that were BLC+ and suspicious for CIS where from patients with previous therapy for urothelial carcinoma and the changes called “suspicious” were therapy related.
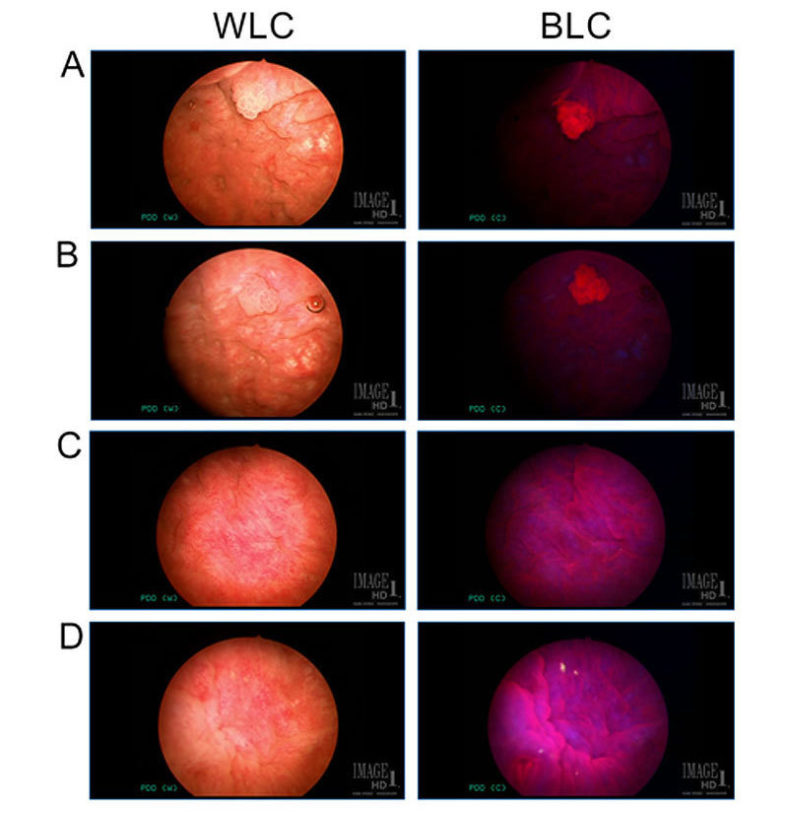
Figure - A&B. White light cystoscopy (WLC) view of an example of a small papillary lesion (left), highlighted by red fluorescence under blue light cystoscopy (BLC; right). Note the background urothelium around the small papillary lesions is seen dark and without fluorescence under BLC. C&D. WLC of flat mucosa of areas that show red fluorescence under BLC (right), indicating to the urologist the area to be biopsied. The pathology of both these examples were benign.
Reference
Pederzoli F, Murati Amador B, Samarska I, Lombardo KA, Kates M, Bivalacqua TJ, Matoso A. Diagnosis of urothelial carcinoma in situ using blue light cystoscopy and the utility of immunohistochemistry in blue light-positive lesions diagnosed as atypical. Hum Pathol. 2019 Aug;90:1-7.
Clinical Significance of Urothelial Carcinoma Ambiguous for Muscularis Propria Invasion on Initial Transurethral Resection of Bladder Tumor.
Urothelial carcinoma that invades the muscle wall (muscularis propria) has a much worse prognosis than those that are non-invasive or invade superficially. While the majority of patients can be confidently diagnosed as superficial or muscle invasive bladder cancer, a small subset of cases are difficult to classify. The purpose of this study was to evaluate the clinical significance of invasive urothelial carcinoma that is ambiguous for muscularis propria invasion (AMP) on initial transurethral resection of bladder tumor (TURBT). We evaluated all consecutive cases that were not able to be classified with TURBT and collected clinical and pathologic information during their follow-up and compared them to patients with muscle invasive disease and those with superficial bladder cancer who underwent radical cystectomy (RC). The great majority of patients with AMP on initial TURBT have advanced disease on RC and emphasizes the need for early repeat TURBT or even consideration of early cystectomy to lower the risk of worse pathological findings and to prolong survival.
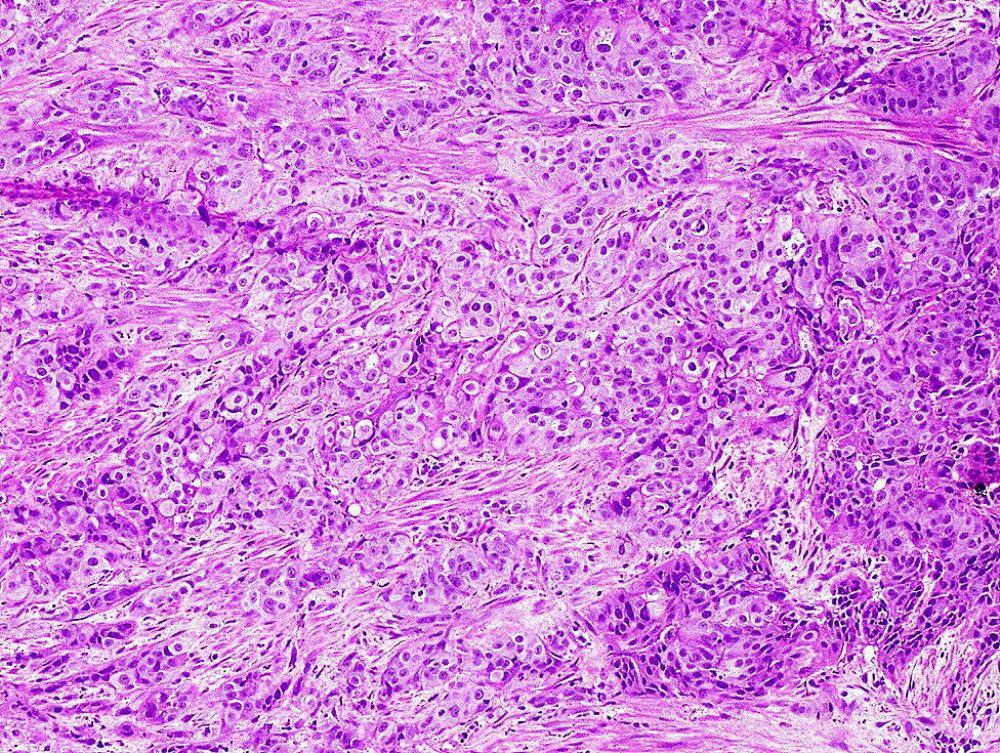
Figure - Invasive urothelial carcinoma splaying thin muscle bundles where it is difficult to know with certainty whether there is muscularis propria invasion.
Reference
Hassan O, Murati Amador B, Lombardo KA, Salles D, Cuello F, Marwaha AS, Daniels MJ, Kates M, Bivalacqua TJ, Matoso A. Clinical Significance of Urothelial Carcinoma Ambiguous for Muscularis Propria Invasion on Initial Transurethral Resection of Bladder Tumor. World J Urol. 2020 Feb;38(2):389-395.
Epithelioid angiosarcoma of the bladder
Primary angiosarcoma of the bladder is very rare, with approximately 30 cases reported in the literature. Those with epithelioid morphology are even rarer, with only single-case reports published. The clinical presentation was hematuria and bladder mass in all cases. Many patients had a history of radiotherapy to the pelvis to treat prostate cancer and to treat uterine cervical cancer. The time from radiotherapy to the diagnosis of epithelioid angiosarcoma ranged from 6 to 15 years. The average size of the tumor was 4 cm. (range, 1 to 8 cm.). Morphologically, the tumors were composed of nests and sheets of highly atypical cells with high nuclear to cytoplasmic ratio, occasional intracytoplasmic lumens, and a hemorrhagic background. None of the cases showed any urothelial carcinoma component. Three patients showed in addition usual angiosarcoma in the resection specimen. The tumor involved the muscularis propria in many patients, and the prostate and seminal vesicles as well. Epithelioid angiosarcoma of the bladder is a rare malignancy that is frequently misdiagnosed as high-grade carcinoma, especially due to positive immunostaining for cytokeratins. This tumor is more frequent in older men with a history of radiotherapy to the pelvis. Morphologic features that should suggest the vascular origin of the tumor include highly atypical nuclei with interspersed erythrocytes, hemorrhagic background, and occasional intracytoplasmic lumens. Patients usually present with muscle invasive disease, and the prognosis is dismal.
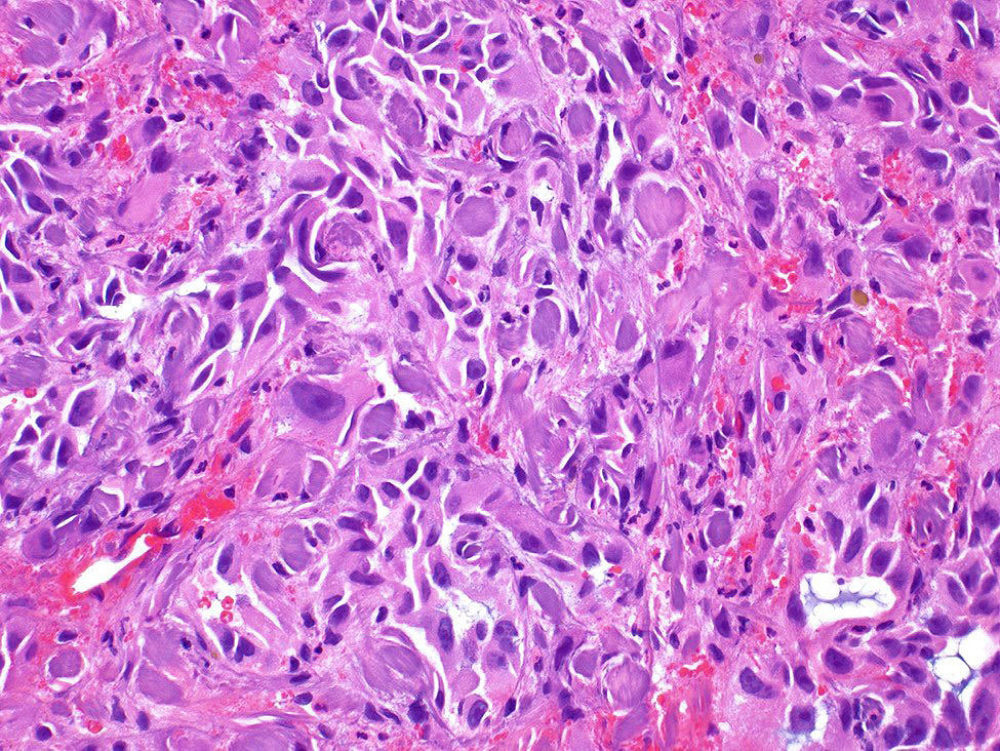
Figure - Epithelioid angiosarcoma of the bladder composed of highly atypical epithelioid cells in a hemorrhagic background.
Reference
Matoso A, Epstein JI. Epithelioid Angiosarcoma of the Bladder: A Series of 9 Cases. Am J Surg Pathol. 2015;39(10):1377-82.