What are the t(6;11) Renal Cell Carcinomas?
Renal cell carcinomas (RCC) harboring the t(6;11) (p21;q12) translocation were first described by Dr. Argani in 2001. While their lineage was initially unclear, the t(6;11) RCC have now been accepted by the 2013 International Society of Urological Pathology Vancouver classification of renal neoplasia and the 2016 World Health Organization Renal Tumor Classification as a subtype of the MiT family of translocation RCC, which includes the more common Xp11 translocation RCC. The t(6;11) translocation fuses the gene for transcription factor EB (TFEB), a transcription factor related to microphthalmia transcription factor (MITF), with Alpha (MALAT1), an untranslated gene of unknown function, resulting in overexpression of native TFEB.
Clinical Features ▼
The t(6;11) RCC are less common than the Xp11 translocation RCC; only approximately 50 cases have been reported in the world literature. While the original cases were reported in children and young adults, it is now clear that these neoplasms can present in adults. The reported age range has ranged from 3 to 68 years. The mean and median age is 31 years, which is significantly lower than that of more common clear cell and papillary RCC of adults. There has been a slight male predominance of cases with a male to female ratio approximately 1.4:1. Similar to the Xp11 translocation RCC, a subset of cases have occurred in patients who have received cytotoxic chemotherapy for other disorders.
Pathologic Features ▼
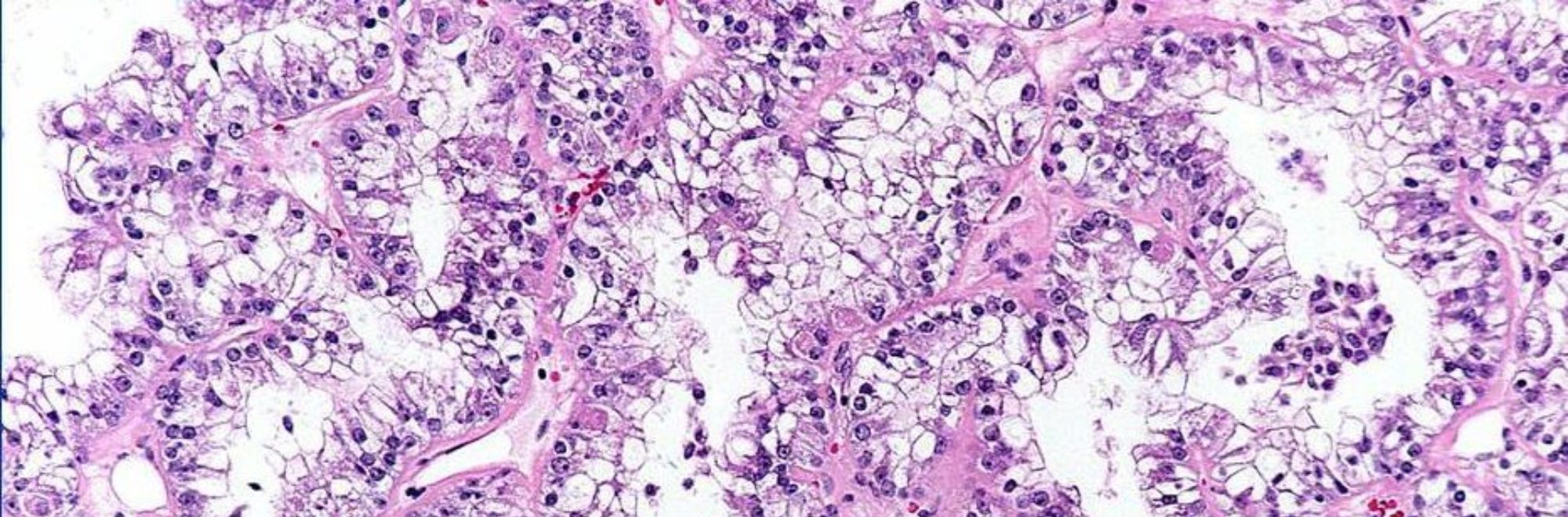
Microscopically, the t(6;11) RCC typically demonstrates a distinctive biphasic morphology, comprising larger epithelioid cells and small cells clustered around basement membrane material. The larger epithelioid cells may have clear to eosinophilic cytoplasm, and their nested architecture is similar to that of clear cell RCC. The smaller cells clustered around basement membrane material resemble the Call-Exner bodies of adult granulosa cell tumor. These neoplasms typically do not show prominent cytologic atypia or mitotic activity. While these lesions appear well delineated grossly, microscopically they characteristically entrap single native renal tubules at their periphery.
Immunohistochemistry ▼
The t(6;11) RCC have a distinctive immunophenotype. These neoplasms express the renal tubular transcription factor PAX8, and most cases at least focally express RCC marker, CD10, and low molecular weight cytokeratin Cam5.2, which supports ultrastructural evidence that these neoplasms demonstrate renal tubular differentiation and thus qualify as RCC. In contrast to other subtypes of RCC, the t(6;11) RCC consistently and diffusely express the melanoma marker Melan A, and consistently demonstrate patchy labeling for HMB45. Unlike malignant melanoma, these neoplasms are negative for MITF and S100 protein. The t(6;11) RCC consistently express the cysteine protease cathepsin K. Other renal tubular markers demonstrate inconsistent immunoreactivity.
The t(6;11)(p21;q12) has been shown to result in a fusion of the intronless, untranslated Alpha gene with TFEB, a gene belonging to the same transcription factor family as MiTF and TFE3. The consequence of the Alpha-TFEB fusion is dysregulated expression of the normal full-length TFEB protein. Along these lines, the t(6;11) RCCs (also known as Alpha-TFEB RCCs) demonstrate specific nuclear labeling for TFEB protein by immunohistochemistry, whereas other neoplasms and normal tissues do not. Hence, nuclear labeling for TFEB is a sensitive and specific diagnostic marker for this neoplasm bearing a TFEB gene fusion, just as nuclear labeling for TFE3 is a sensitive and specific marker for neoplasms bearing TFE3 gene fusions. More recently, a break-apart fluorescence in situ hybridization (FISH) assay to detect TFEB gene rearrangements has been validated on formalin-fixed tissues, and appears to be superior to the TFEB IHC assay. The TFEB break-apart FISH assay is less affected by variable fixation than the TFEB immunohistochemical assay, and thus represents the preferred diagnostic test for establishing the diagnosis of t(6;11) RCC in formalin-fixed, paraffin embedded material.
The differential diagnosis for t(6;11) RCC is broad, reflecting the variable morphology which genetically confirmed cases may demonstrate. In fact, Dr. Argani has seen cases in adults which overlap greatly with clear cell RCC, such that the author would have not suspected the diagnosis of t(6;11) RCC had immunohistochemistry not been performed. Since standard practice is not to perform immunohistochemistry on adult RCC which are morphologically consistent with clear cell RCC, some adult MiT family translocation carcinomas are likely misclassified as clear cell RCC in daily practice.
Outcome ▼
The t(6;11) RCC have generally been more indolent neoplasms than the Xp11 translocation RCC. Of the approximately 50 cases in the published literature, only four have developed metastases, leading to patient death in three cases. Most neoplasms have presented at low stage (pT1 or pT2) and have had benign follow-up. Like the Xp11 translocation RCC, these neoplasms have demonstrated the capacity to recur late (up to eight years after diagnosis), so long term follow-up is important for these patients.