What are Xp11 translocation renal cell carcinomas?
Xp11 translocation renal cell carcinomas (RCCs) are a distinctive subtype of RCC characterized by chromosomal translocations with breakpoints involving the TFE3 transcription factor gene, which maps to the Xp11.2 locus. The result is a fusion of the TFE3 transcription factor gene with one of multiple reported genes including ASPSCR1 ( ASPL), PRCC, NonO (p54nrb), SFPQ (PSF), and CLTC (Table 1). The three most common Xp11 translocation RCCs are those bearing the t(X;1)(p11.2;q21) which fuses the PRCC and TFE3 genes, the t(X;17)(p11.2;q25) which fuses the ASPSCR1 and TFE3 genes , and the t(X;1)(p11.2;p34) which fuses the SFPQ (PSF) and TFE3 genes. The ASPSCR1-TFE3 gene fusion is the same gene fusion found in alveolar soft part sarcoma (ASPS), a rare pediatric neoplasm of uncertain histogenesis. However, the translocation in Xp11 translocation RCC is balanced, which may contribute to the differences seen at the clinical and histopathologic levels between Xp11 translocation RCC and ASPS. Other reported but rare translocations are the inv (X)(p11.2;q12) which fuses the NonO (p54nrb) and TFE3 genes; the t(X;17)(p11.2;q23) which fuses the CLTC and TFE3 genes5 , and the t(X;3)(p11.2;q23)6 which fused the PARP14 and TFE3 genes. Variant translocations with no known fusion partner include t(X;10)(11.2;q23).
Clinical Features ▼
Xp11 translocation RCC were first recognized in children. Although RCC accounts for less than 5% of renal neoplasms in children, Xp11 translocation RCCs constitute a significant percentage of these cases. Approximately 40% of pediatric RCC have been classified as Xp11 translocation RCC, with a range from 20% to 75% of pediatric RCC cases among different series. The higher frequencies have generally come from single institution series, which may be less biased than multi-institution, tumor-repository based series.
The frequency of Xp11 translocation RCC in adults may be underestimated, perhaps due to morphological overlap with more common adult RCC subtypes, such a conventional clear cell RCC and papillary RCC. The frequency ranges from 1-4% in different studies. While Xp11 translocation RCC is therefore on a percentage basis rare in adults, RCC is overall much more common in adults than in children. If there are approximately 30,000 new cases of RCCs in adults each year, 4.2 % of 30,000 cases would total 1,260 adult Xp11 translocation RCC per year. In contrast, 40% of the 25 pediatric RCC in the United States would total 10 pediatric Xp11 translocation RCC per year. Thus, adult Xp11 translocation RCC may outnumber pediatric Xp11 translocation RCC by orders of magnitude due to the much higher incidence of RCC in the adult population.
Prior exposure to cytotoxic chemotherapy is currently the only known risk factor for development of Xp11 translocation RCC: up to 15% of patients with these tumors had a history of prior chemotherapy exposure. Indications for chemotherapy have included Wilms tumor, Ewing sarcoma, systemic lupus erythematosus (SLE), acute leukemia, and bone marrow transplant. The post-chemotherapy interval ranged from 4-13 years, though more recent studies have documented occurrence of Xp11 translocation RCC within 2 years of chemotherapy. All reported patients received either a DNA topoisomerase II inhibitor and/or an alkylating agent. Although they have differing mechanisms of action, both cytotoxic agents break DNA, which may initiate repair or recombination mechanisms that permit a chromosome translocation to occur.
Pathologic Features ▼
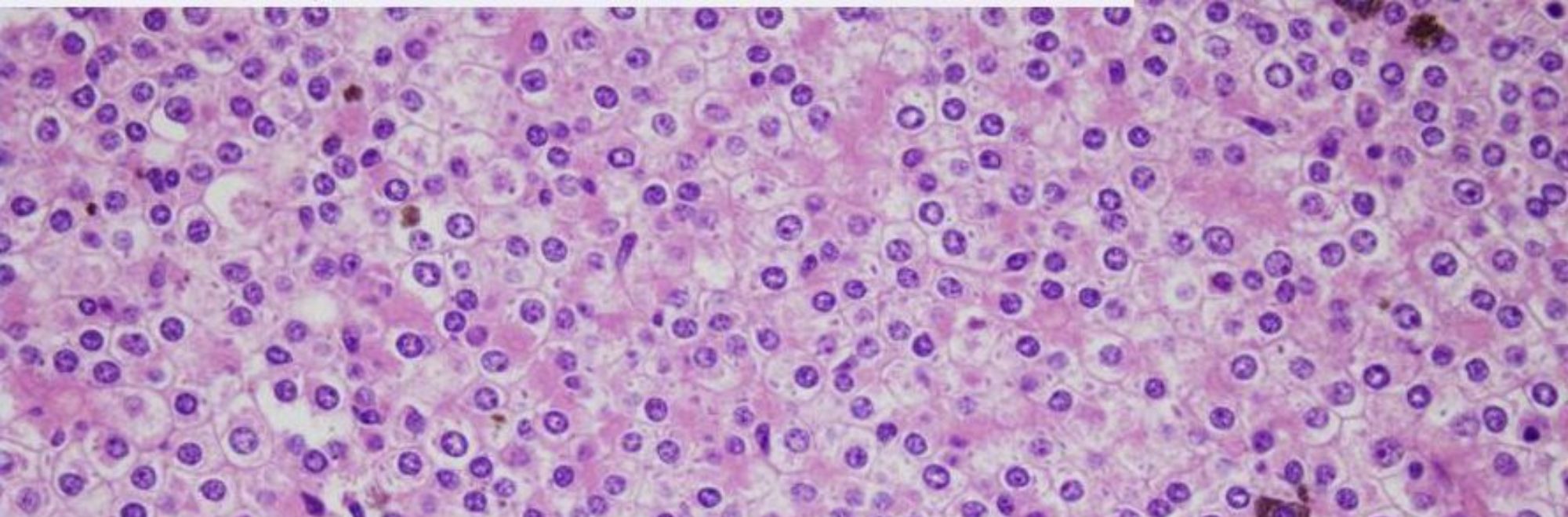
Microscopically, the most distinctive histologic pattern of Xp11 translocation RCC is that of a neoplasm featuring papillary architecture and epithelioid clear cells. In some cases, abundant psammoma bodies can be seen. Xp11 translocation RCCs can also present with unusual morphology mimicking other types of RCCs. The wide spectrum of morphology seen in Xp11 translocation RCCs emphasizes the need to consider these carcinomas in the differential diagnosis of unusual, difficult to classify RCC occurring in both children and adults.
Immunohistochemistry ▼
In contrast to most RCC, Xp11 translocation RCC underexpresses epithelial markers such as cytokeratins and EMA. Vimentin is often negative. In contrast, Xp11 translocation RCC do consistently express CD10 and RCC marker as well as PAX2 and PAX8, similar to both clear cell RCC and papillary RCC. Occasionally, Xp11 translocation RCCs may express melanocytic markers such as Melan-A and HMB-45, particularly in cases associated with less common gene fusions.
The most sensitive and specific immunohistochemical marker for the Xp11 translocation RCC is strong nuclear TFE3 immunoreactivity using an antibody to the C-terminal portion of TFE3. The fusion products found in Xp11 translocation RCC retain the C-terminal portion of the TFE3 transcription factor, including its DNA binding domain, leucine-zipper dimerization domain and nuclear localization signal. Although native TFE3 protein is ubiquitously expressed, its normal levels are generally undetectable by immunohistochemistry. However, the TFE3 gene fusion partners in the Xp11 translocation RCC contribute strong promoters leading to overexpression of the fusion protein and strong nuclear labeling for TFE3 by immunohistochemistry. Adjacent renal tubules and stroma should be negative by this assay. When properly performed with overnight antibody incubation, the TFE3 immunohistochemical assay is very sensitive and specific for neoplasms bearing TFE3 gene fusions. Using moderate and strong immunoreactivity as a positive result, the initial study found sensitivity of 97.5% (39 of 40 of positive control tumors) and specificity of 99.6 % (6 of 1476 negative controls). However, a major drawback is that the assay is technically challenging, and suboptimal fixation can permit detection of native TFE3 protein resulting in high background staining. TFE3 break-apart fluorescence in situ hybridization (FISH) assays can provide molecular confirmation of Xp11 translocation RCC in paraffin embedded tissue. Hybridization with probes centromeric and telomeric to TFE3 normally yields a fusion signal but TFE3 rearrangement results in a split signal. The TFE3 break-apart FISH assay has proven to be very useful for detecting TFE3 gene fusions in Xp11 translocation RCC, and suffers less from the technical and fixation issues associated with the TFE3 immunohistochemical assay.
Cathepsin K is also useful as a specific marker for the MiT family translocation RCC. Expression of cathepsin K is mediated by expression of MiTF in osteoclasts. Since TFE3 overlaps functionally with MiTF, overexpressed TFE3 fusion proteins in the Xp11 translocation RCC are hypothesized to function like MiTF and mediate cathepsin K expression. Among cytogenetically confirmed Xp11 translocation RCCs, approximately 60% label for cathepsin K; interestingly, PRCC-TFE3 RCC label more frequently for cathepsin K than do the ASPSCR1-TFE3 RCC in the limited number of cases tested. In contrast, none of the conventional clear cell RCCs (210 cases), papillary RCCs (40 cases), chromophobe RCCs (25 cases), oncocytomas (30 cases) or adjacent non-neoplastic renal tissue showed immunoreactivity for cathepsin K. t(6;11) RCC also consistently express cathepsin K, likely due to the similar effects of overexpressed native TFEB (see below). Hence, cathepsin K can be considered highly specific for the diagnosis of an MiT family translocation RCC. However, the sensitivity is less than that of TFE3 IHC or FISH.
Outcome ▼
Since Xp11 translocation RCC was recognized as a distinct entity by the WHO only 10 years ago, outcome data on Xp11 translocation RCC are still premature at this time. Outcomes have been highly variable, with some patients surviving decades with indolent disease and others dying rapidly of progressive disease. Overall, survival is similar to that of patients with clear cell RCC, and significantly worse than those of patients with papillary RCC. Ellis et al. reviewed the published literature on Xp11 translocation RCC with the ASPSCR1-TFE3 and PRCC-TFE3 gene fusions. In multivariate analysis, only advanced stage (specifically distant metastasis) and older age at diagnosis independently predicted death. Fusion subtype impacted presentation; ASPSCR1-TFE3 RCC were more significantly more likely to present with regional lymph node metastasis (24 of 32 evaluable cases, 75%) than were PRCC-TFE3 RCC (5 of 14 cases, 36%); however, most of the former patients remained disease free without adjuvant therapy. Hence, locally advanced stage may not predict adverse outcomes. However, all patients who presented with distant metastases had ASPSCR1-TFE3 RCC and these patients have poor outcomes.
Children with locally-advanced disease (regional nodal metastases but without hematogenous spread) have a favorable short-term prognosis. In a literature review, over 90% of these patients remained disease free at last follow-up having a median of 4.4 years and a mean of 6.3 years. However, likely reflecting their usually low proliferative indices, Xp11 translocation RCC have the potential to metastasize late, as many as 20 or 30 years after diagnosis. Therefore, good long-term follow up data are necessary before any favorable short-term outcome can be confirmed. Adults do poorly when presenting with systemic metastases. Unfortunately, Xp11 translocation RCCs in adults often present this way. Mean survival after diagnosis in one study was 18 months with a range of 10-24 months. Another adult series with at least one year of follow-up reported that five of six patients developed hematogenous spread and two patients died within a year of diagnosis.
Xp11 Translocation PEComa and Melanotic Xp11 Translocation Renal Cancer
A subset of perivascular epithelioid cell tumors (PEComa) in extra-renal sites have recently been shown to harbor TFE3 gene fusions. Although the cases are few (less than 10 cases reported in the literature), distinctive features included young age, absence of association with tuberous sclerosis, minimal muscle marker immunoreactivity, and prominent epithelioid clear cell morphology. Most cases have demonstrated an SFPQ(PSF)-TFE3 gene fusion.
Another unusual malignant melanotic epithelioid cell renal neoplasm bearing a TFE3 gene fusion has also been reported. The two initial cases occurred in children and presented with widespread metastatic disease. The neoplastic cells were Melan-A and HMB-45 positive but negative for cytokeratins, renal tubular markers and muscle markers. Most cases have demonstrated an SFPQ(PSF)-TFE3 gene fusion. Around 10 total cases have now been reported, but Dr. Argani has seen more in his unpublished consultation material. These neoplasms are difficult to classify, overlapping with Xp11 translocation RCC, melanoma and perivascular epithelioid cell neoplasm (PEComa). While they are immunoreactive to TFE3, all reported cases were negative for renal tubular markers CD10, PAX2 and PAX8, in contrast to the typical Xp11 translocation RCC. The neoplastic cells are not immunoreactive to MiTF, in contrast to melanoma. PEComas represent the most closely associated phenotype. Both melanotic Xp11 translocation cancer and Xp11 translocation PEComas may represent distinct entities which overlap with Xp11 translocation RCC, and broaden the spectrum of TFE3-associated cancers.