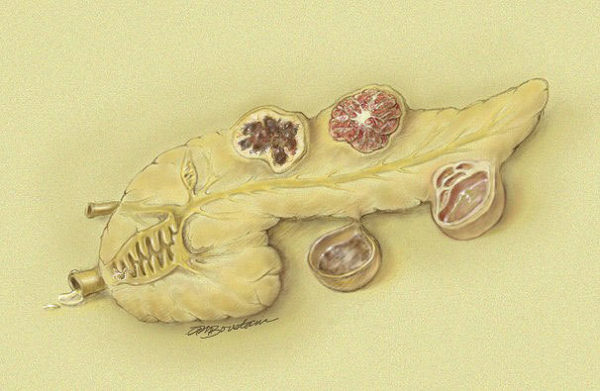
Learn about the characteristics of five different cysts: Serous Cystic Neoplasm, Mucinous Cystic Neoplasm, Pseudocyst, Solid-Pseudopapillary Neoplasm and IPMN.
Five Helpful Hints for Diagnosing Pancreas Cysts
1. What is the patient's gender?
Some cystic neoplasms, including mucinous cystic neoplasms and solid-pseudopapillary neoplasms, are much more common in women. Pseudocysts, because of there association with alcoholism, tend to be more common in men.
2. Do the cysts communicate with the large pancreatic ducts?
This feature can help distinguish intraductal papillary mucinous neoplasms, which do communicate with the ducts, from mucinous cystic neoplasms, which do not.
3. What are the cyst contents?
The cysts of mucinous cystic neoplasms and intraductal papillary neoplasms contain thick tenacious mucoid material. The cysts of serous cystic neoplasms, as the name suggests, contain thin straw colored fluid. Pseudocysts and solid-pseudopapillary neoplasms can contain necrotic/hemorrhagic debris.
4. What type of cells, if any, line the cysts?
Pseudocysts lack an epithelial lining. Mucinous cystic neoplasms and intraductal papillary neoplasms are lined by tall columnar mucin-producing cells. Serous cystic neoplasms are lined by low cuboidal glycogen-rich cells.
5. What is the character of the stroma?
Mucinous cystic neoplasms have a distinctive "ovarian-type" of dense stroma. The often cystic lesions do not.
Serous Cystic Neoplasm
Definition and Helpful Hints ▼
Key Points - Benign; Associated with von Hippel-Landau Syndrome
Serous Cysts are epithelial neoplasms composed of uniform cuboidal glycogen-rich cells that usually form numerous small cysts containing serous fluid. Almost all are benign, however, rare malignant serous cystadenocarcinomas have been reported.
Serous cystadenomas (SCA) have also been know as: microcystic adenoma, serous microcystic adenoma, macrocystic SCA, serous oligocystic and ill-defined adenoma, and glycogen-rich cystadenoma. Serous cystadenomas are associated with von Hippel-Landau syndrome (VHL), an autosomal dominant disorder characterized by hemangioblastomas of the central nervous system (CNS) and retina, renal neoplasms and cysts, and pheochromocytomas. The pancreatic lesions attributed to VHL may develop earlier than CNS lesions, thereby indicating VHL.
| Patient gender | Location in Pancreas | Communication with ducts | Cyst contents | Epithelial lining | Stromal character |
|---|---|---|---|---|---|
| mostly female | head/body | none | thin straw colored fluid | cuboidal | delicate and vascular to fibrous |
Clinical ▼
Symptoms - Abdominal pain; Dyspepsia; Nausea; Fever; Vomiting
Serous cystic neoplasms are twice as likely in women as men. Patient age ranges from 18-91years, with a median age of 70.
25-30% of patients are asymptomatic, however, most patients present with symptoms such as: abdominal or epigastric pain, dyspepsia, nausea, vomiting, fever , melena, or weight loss. Abdominal masses may be found upon physical examination.
In rare cases, patients develop complications from serous cysts. Symptoms from these complications include: left-sided portal hypertension due to splenic vein occlusion, obstructive jaundice, acute abdomen with hematoperitoneum due to rupture of the tumor, erosion vessels in and around the cyst, recurrent pancreatitis, ulceration of the duodenum causing GI hemorrhage, or Evan's syndrome (autoimmune hemolytic anemia and immune thrombocytopenia).
Gross ▼
Key points - Located anywhere in pancreas; No communication with ducts; Sponge-like appearance
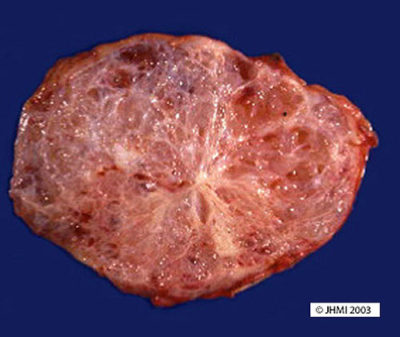
Serous cystadenomas can be located anywhere in the pancreashead, body, or tail. Communication with pancreatic ducts is not normally a finding with serous cysts, although there have been a few reported. They are normally single lesions. Multifocal lesions seem to be associated with VHL.
Most cystadenomas are large (mean 6.0 cm), well-demarcated, somewhat bosselated masses, composed of innumerable small (usually <2mm) thin-walled cysts, imparting a sponge-like appearance on cross-section. A stellate scar appears in the center of the neoplasms and is often calcified. The cysts can be larger in the periphery of the neoplasm. Rare serous cystadenomas composed of only a few larger cysts (oligocystic variant) and a case of a serous cystadenoma with a solid growth pattern (solid serous cystadenoma) have been reported.
Histological ▼
Key Points - Single layer of clear cuboidal cells; Abundant glycogen; No mucin
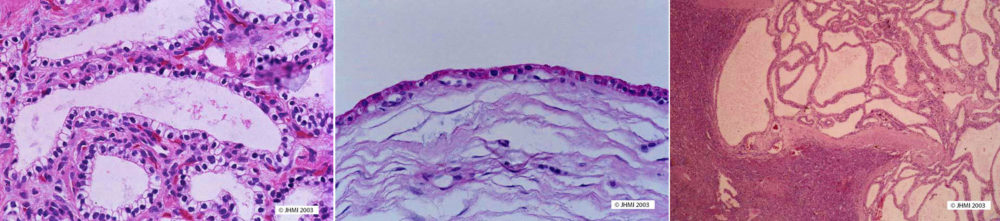
Cysts in serous cystadenomas are composed of a single layer of clear cuboidal cells. These cells usually form flat sheets that line the cysts and only rarely form papillae that project into the cystic spaces. The neoplastic cells have clear cytoplasm because they contain abundant intracytoplasmic glycogen. They do not produce mucin. The nuclei are small, round, and have uniform chromatin and inconspicuous nucleoli. Atypia and mitoses are essentially absent. The central stellate scar and the stroma separating the cysts are composed mostly of relatively acellular collagenous connective tissues, but the stroma may contain entrapped islets of Langerhans and acini.
Special stains will highlight the abundant glycogen and absence of mucin. A periodic acid Schiff (PAS) stain will be strongly positive and sensitive to diastase digestion. Stains for mucin, including a mucicarmine and alcian blue stains, will be negative, as will stains for neuroendocrine differentiation including the Gremelius stain.
Radiological ▼
Key Points
CT, MRI, EUS, MRCP - Sunburst pattern of calcification
Enhanced CT and Angiography - Hypervascularity
Although preoperative diagnosis of serous cystic neoplasms relies on CT and EUS images, neither modality, used independently or together, provides a definitive diagnosis.
Plain CT shows a honeycomb pattern of microlacunae, with thin septae separating different segments. Serous cystic neoplasms can have a sunburst pattern of central calcification, seen in 10-30% of cases. Peripheral calcification may be seen in some cases, leading to a differential diagnosis of mucinous cystic neoplasm or pseudocyst. MRI, EUS, and magnetic resonance cholangiopancreatography also show this same honeycomb pattern. Hypervascularity, a defining characteristic of serous cysts, can be seen through angiography and enhanced CT.
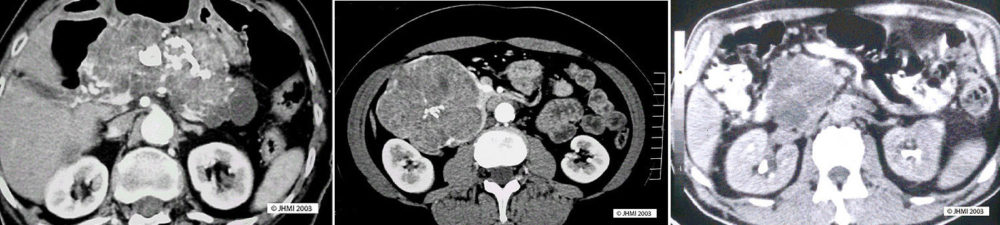
Differential Diagnosis ▼
Key Points - Mucinous cystic neoplasm
Given the characteristic gross and microscopic appearance of serous cystadenomas, the diagnosis is usually relatively easy and straightforward. Nonetheless, establishing the correct diagnosis of a cystic lesion in the pancreas is important for clinical management and a broad differential diagnosis should be kept in mind.
Mucinous cystic neoplasms, because of their significantly greater malignant potential, are probably the most important neoplasms to consider in the differential diagnosis. In particular, serous oligocystic cystadenomas, because they are composed of larger cysts, can grossly mimic mucinous cystic neoplasms. While both serous and mucinous cystic neoplasms will be cystic, the cysts in mucinous cystic neoplasms tend to be larger and are filled with thick tenacious fluid, not the clear watery fluid of serous cystadenomas. At the microscopic level mucinous cystic neoplasms will be lined by tall columnar cells containing abundant mucin, while the lining in serous cystadenomas is cuboidal and glycogen-rich. Mucicarmine and other stains for mucin will stain mucinous cystic neoplasms and not serous cystadenomas. Both neoplasms will express cytokeratin, but only the mucinous cystic neoplasms will label with antibodies to carcinoembryonic antigen. In cases in which the epithelium is extensively denuded, the presence of 'ovarian-type' stroma can be used to establish the diagnosis of a mucinous cystic neoplasm.
References ▼
Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol. 1978 Mar; 69(3):289-98.
Mucinous Cystic Neoplasm
Definition and Helpful Hints ▼
Key Points - Uncommon; Mostly in females; "Ovarian-type" stroma
Mucinous cystic neoplasms are neoplasms composed of mucin-producing epithelial cells associated with an ovarian-type of stroma. The neoplastic epithelial cells form one or more cysts that are filled with mucoid fluid and these cysts usually do not communicate with the larger pancreatic ducts. Invasive mucinous cystadenocarcinomas are mucinous cystic neoplasms associated with an invasive carcinoma, while non-invasive mucinous neoplasms can be categorized into adenomas, borderline neoplasms and carcinoma in situ based on the degree of architectural and cytological atypia of the epithelial cells.
| Patient gender | Location in Pancreas | Communication with ducts | Cyst contents | Epithelial lining | Stromal character |
|---|---|---|---|---|---|
| Female | Tail | None | Thick, tenacious mucoid material | Tall, columnar | "Ovarian-type" |
Clinical ▼
Symptoms - Abdominal pain or discomfort
Mucinous cystic neoplasms are relatively uncommon. They account for only 5.7% of all primary pancreatic tumors seen in consultation at the Armed Forces Institute of Pathology. In some reported series of mucinous cystic neoplasms all of the patients have been female, however, well-documented cases have been reported in men, and a female to male ratio of 90:10 is probably reasonable. The mean age at diagnosis is between 40 to 50 years with a range of 14-95 years. In most series of mucinous cystic neolasms, patients with mucinous cystadenocarcinomas are 5 to 10 years older than the patients with benign mucinous cystic neoplasms.
Mucinous cystic neoplasms have not been associated with any genetic syndromes.
Most patients with mucinous cystic neoplasms present with vague abdominal symptoms that include epigastric pain or a sense of abdominal fullness.Less commonly, the patients develop gastrointestinal symptoms including nausea and vomiting, diarrhea, anorexia, and weight loss. As many as 20% of mucinous cystic neoplasms are discovered incidentally during abdominal imagingfor an unrelated indication. Only a small minority of the patients present with jaundice. Those that do present with jaundice usually have mucinous cystadenocarcinomas involving the head of the gland. Mucinous cystic neoplasms can sometimes be palpated in the left upper quadrant on physical examination.
Gross ▼
Key Points - Body or tail of pancreas; Multilocular; No communication with ducts
The majority (70-90%) of mucinous cystic neoplasms arise in the body or tail of the pancreas, and only a minority (10-30%) involve the head of the gland. Mucinous cystadenocarcinomas involve the head of the gland slightly more often than do mucinous cystadenomas. Mucinous cystic neoplasms are usually quite large (2-35 cm, mean ~7 to 10 cm), and mucinous cystadenocarcinomas tend to be even larger.
On cut section mucinous cystic neoplasms are usually multilocular, but an occasional tumor can be unilocular, a feature that can mimic a pseudocyst. The cysts are typically between 1 and 3 cm, but cysts as small as a few millimeters and as large as 23 cm have been reported. The cysts have a thick-wall and are filled with thick tenacious mucoid material. The lining of the cysts can be smooth, particularly in benign mucinous cystic neoplasms, or there can be intracystic papillary excrescences (projections) and even solid mural nodules; features that suggest the diagnosis of a mucinous cystadenocarcinoma. Focal calcifications are sometimes present in the periphery of the tumors. Only a small minority (~5%) communicate with the larger pancreatic ducts.
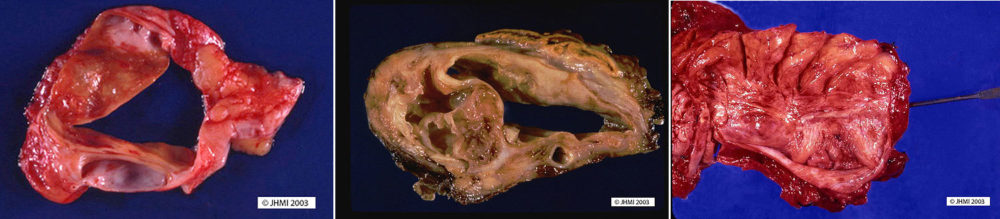
Histological ▼
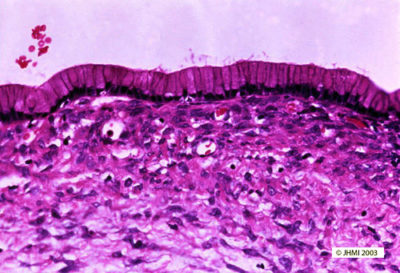
Key Points - Tall, columnar mucin-producing epithelium; "Ovarian-type" stroma
The cysts of mucinous cystic neoplasms, as one would expect from their gross appearance, usually do not communicate microscopically with the pancreatic ducts. The cysts are lined by tall columnar mucin-producing epithelium. These columnar cells have basal nuclei and abundant intracytoplasmic apical mucin, and can form flat sheets or papillae. The epithelium is often focally denuded and several sections may be needed to demonstrate an epithelial lining. The epithelium can be quite bland with uniform small basally placed nuclei, or the epithelium can show significant architectural and cytological atypia with cribriforming, an increased nuclear to cytoplasm ratio, loss of nuclear polarity and pleomorphism. Most remarkably, in a single neoplasm there is often an abrupt, at times striking, transition from completely bland epithelium to epithelium with sigificant atypia.
The walls of the cysts contain a very distinctive "ovarian-type" stroma. This stroma is composed of densely packed spindle cells with sparse cytoplasm and uniform elongated nuclei. The stroma can be partially hyalinized in some mucinous cystic neoplasms, and it is not unusual for the stroma to contain entrapped normal pancreatic tissue including islets of Langerhans and acini.
Non-invasive mucinous neoplasms are categorized into adenomas, borderline neoplasms and carcinoma in situ based on the degree of architectural and cytological atypia of the epithelial cells. Mucinous cystic neoplasms should be categorized based on the worst degree of atypia present in the lesion.
Approximately, one-third of all mucinous cystic neoplasms are associated with invasive carcinoma. These invasive carcinomas are usually a tubular/ductal type of invasive adenocarcinoma. Unidfferentiated carcinomas with osteoclast-like giant cells, invasive adenosquamous carcinomas and colloid carcinomas have also been reported. The diagnosis of an invasive carcinoma should only be made when there is clear-cut invasion into the stroma. This invasion is often associated with a desmoplastic reaction. The invasive and in situ carcinomas in mucinous cystic neoplasms can be very focal. A benign diagnosis cannot be established on biopsy alone, nor can it be established in an adequately examined resected specimen.
Mucicarmine and PAS stains will confirm the presence of substantial quantities of intracellular and extracellular mucin. These are predominantly sulphated acid mucins with some neutral mucins; two-thirds of the neoplasms are alcian blue positive. Gremilius/Churukin-Schenk (argyrophilic) and Fontana-Masson (argentafin) stains will stain scattered endocrine cells at the base of the epithelial cells.
Radiological ▼
Key Points
CT, EUS - Thick-walled cystic mass; Thick pseudocapsule
Computerized tomography (CT) scans will usually demonstrate a well-demarcated thick-walled cystic mass. The cysts are usually large (1-3 cm) and, peripheral calcifications can be seen within the capsule of the neoplasm. Mural nodules and papillary excrescences into the cysts are more common in cystadenocarcinomas.
Ultrasound will also demonstrate the cystic nature of these neoplasms and ultrasound has the advantage that it can be used to facilitate biopsy of the lesion and sampling of the larger than benign mucinous cystic neoplasms. The outer surface of these tumors is usually well-demarcated, and the neoplasms often have a thick pseudocapsule.
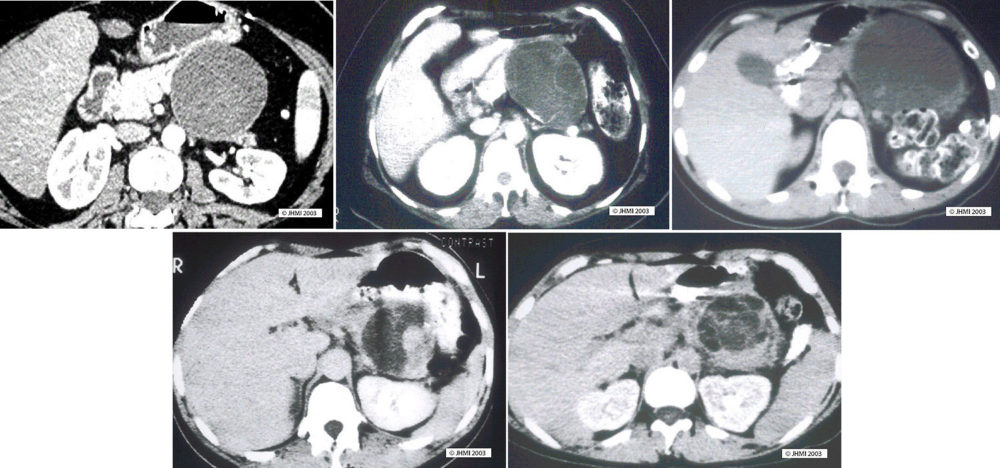
Differential Diagnosis ▼
Key Point - Serous Cystic Neoplasms
Mucinous cystic neoplasms and pseudocysts can both produce cystic masses with thick-walled large cysts; however, these two entities can be distinguished from each other if one considers the clinical, gross and microscopic findings. Pseudocysts are more common in men than in women and the patients typically have a history of pancreatitis and elevated serum amylase levels. By contrast, mucinous cystic neoplasms are more common in women than in men, most of the patients do not develop pancreatitis, and these patients usually have normal serum amylase levels. Most pseudocysts are unilocular and actually extrapancreatic, while most mucinous cystic neoplasms are multilocular and involve the body/tail of the gland. Pseudocysts contain necrotic/hemorrhagic debris with high amylase levels, while the cysts of mucinous cystic neoplasms contain tenacious mucoid material with amylase levels equal to or lower than the patient's serum amylase level. Microscopically pseudocysts will lack an epithelial lining, while mucinous cystic neoplasms will be lined by columnar mucin-containing epithelial cells. Finally, even when the epithelium of a mucinous cystic neoplasm is partially denuded, the presence of ovarian-type stroma will help separate mucinous cystic neoplasms from pseudocysts.
Mucinous cystic neoplasms also need to be distinguished from intraductal papillary neoplasms. IPMN's occur more often in men than in women and tend to involve the head of the gland more frequently than the tail of the gland. By contrast, mucinous cystic neoplasms occur much more commonly in women, and involve the body/tail more frequently than the head of the gland. IPMN's, by definition, involve a larger pancreatic duct, while the cysts in vast majority of mucinous cystic neoplasms do not communicate with the larger ducts. Microscopically, the cysts of both neoplasms will be lined by columnar mucinous epithelium, but only mucinous cystic neoplasms will have the dense ovarian type stroma.
It is important to distinguish between mucinous and serous cystic neoplasms because almost all serous cystadenomas are benign, while mucinous cystic neoplasms have a significant malignant potential. The smaller size of the cysts, central stellate scar and low cuboidal lining make it easy to distinguish most serous cystadenomas from mucinous cystic neoplasms. Oligocystic serous cystadenomas, because they are composed of only a few larger cysts, can mimic mucinous cystic neoplasms, the cyst contents, epithelial lining and the strom can help distinguish between these two entities.
Both solid-pseudopapillary tumors and mucinous cystic neoplasms occur much more commonly in women than they do in men. Both of these entities should therefore be near the top of the differential diagnosis of cystic neoplasms in women. It is relatively easy to distinguish between these two entities. The spaces in solid-pseudopapillary tumors are not true cysts; instead they are areas of necrosis and hemorrhage with drop-out of the neoplastic cells. By contrast, the cysts in mucinous cystic neoplasms will be filled with thick tenacious mucoid material. Microscopically, solid-pseudopapillary tumors are composed of relatively uniform cells with granular eosinophilic cytoplasm and uniform oval nuclei. Mucinous cystic neoplasms are line by a well-oriented layer of mucin comatining columnar epithelial cells. Mucinous cystic neoplasms will express carcinoembryonic antigen and MUC5AC, while solid-pseudopapillary tumors will express CD 10 and vimentin, alph-1-antitrypsin, and show an abnormal nuclear accumulation of beta-catenin.
References ▼
Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978 Jun; 69 (6): 573-80
Pseudocyst
Definition and Helpful Hints ▼
Key Points - Most common cyst type; Also known as "postnecrotic pseudocyst"; Result of acute or chronic pancreatitis
Pseudocysts are grossly visible and well-demarcated cystic lesion, which contain necrotic-hemorrhagic material and/or turbid fluid rich in pancreatic enzymes. The cystic contents are enclosed by a wall of inflammatory and fibrous tissue devoid of an epithelial lining. Pseudocysts are due to extensive confluent autodigestive tissue necrosis caused by alcoholic, biliary, or traumatic acute pancreatitis, that is why they are also called postnecrotic pseudocysts.
Pseudocysts are the most common pancreatic cyst type. They account for approximately 75% of pancreatic cystic lesions. They are usually a result of acute or chronic pancreatitis. Whereas many fluid collections related to acute pancreatitis tend to resolve spontaneously pseudocysts can develop when an intra- or extrapancreatic fluid collection is walled off. The incidence of pseudocysts following acute pancreatitis has been estimated at 2-3%.
| Patient gender | Location in Pancreas | Communication with ducts | Cyst contents | Epithelial lining | Stromal character |
|---|---|---|---|---|---|
| Male | Extrapancreatic | None | Necrotic | None | Fibrotic tissue |
Clinical ▼
Symptoms/Associated with - Abdominal pain; Alcoholism in me; Trauma in younger patients
In the United States, almost two-thirds of pseudocyst cases are related to alcoholism, 15% with biliary disease, and 13% are idiopathic, and the rest are due to operative and non-operative trauma, hyperlipidemia, and hereditary pancreatitis.
Pseudocysts develop in people of all ages. In general, however, pseudocysts in older patients are a sequela of alcoholic and biliary pancreatitis, while pseudocysts in younger patients are a result of heredity or trauma. An almost equal sex distribution is observed in the nonalcoholic pancreatitis group, whereas a strong prevalence of men is found in alcoholic pancreatitis. More than half of the patients with alcoholic chronic pancreatitis develop a pseudocyst; in almost 90% of the cases this occurs within the first 6 years from the onset of the disease.
Gross ▼
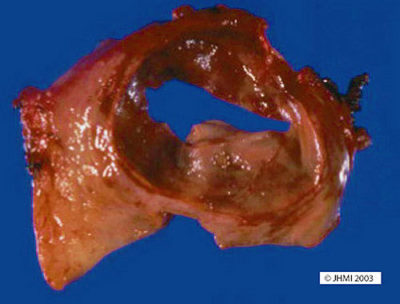
Key Points - Anywhere in pancreas, many extrapancreatic; Occasional communication with ducts; Filled with necrotic debris
Pseudocysts can occur anywhere in the pancreas: head, body, or tail. Many, in fact, are extrapancreatic. They vary in size as well, anywhere from 3-20 cm. The largest cysts are found in alcoholic pancreatitis and are located outside the pancreas, often lying in the lesser omental sac. Other locations are the retroperitoneum between the stomach and transverse colon, the stomach, and the liver, and the perirenal space or the subdiaphragmatic space.
Pseudocysts may sometimes communicate with the pancreatic duct system. They are usually filled with necrotic-hemorrhagic material and/or turbid fluid rich in pancreatic enzymes.
Histological ▼
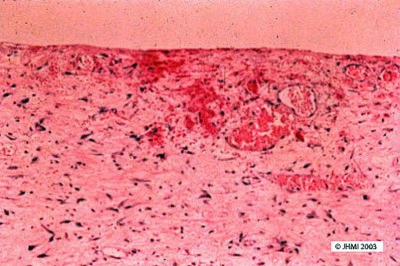
Key Points - Fibrin wall; No epithelial lining
The wall of a pseudocyst consists of fibrin, granulation tissue and loose fibrotic tissue. It does not have an epithelial lining. The adjacent pancreas often shows changes of acute and/or chronic pancreatitis.
Radiological ▼
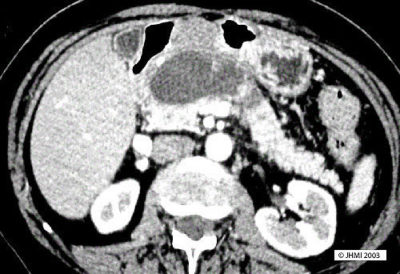
Key Points - Thick well-defined wall; Fine Needle Aspiration; Granular debris and lipid droplets
CT and ultrasonography are usually effective in the diagnosis of pseudocysts. A CT/ultrasonography guided fine needle aspiration biopsy shows material consisting of granular debris and lipid droplets, and a few inflammatory cells such as neutrophils, macrophages, and a few plasma cells. The fluid characteristically has a high amylase level.
On plain CT, pseudocysts appear as a collection of fluid and show a thick well-defined wall. Pseudocysts that do not appear homogenous or show an increase in attenuation, may reveal hemorrhage or infection. ERCP and MRCP can be useful since they can display the relations between the pancreatic duct and the pseudocysts.
Differential Diagnosis ▼
Key Points - Mucinous Cystic Neoplasm; Solid-pseudopapillary Neoplasm
The gross appearance of a pseudocyst resembles that of a mucinous cystic tumor and a solid-pseudopapillary tumor. The absence of any epithelial lining or epithelial tissue in pseudocysts excludes the diagnosis of a cystic neoplasm. In addition, mucinous cystic tumor and solid-pseudopapillary tumor predominantly occur in women, whereas pseudocysts are associated with a history of pancreatitis and usually chronic alcoholism and occur more often in men.
Solid-Pseudopapillary Neoplasms
Definition and Helpful Hints ▼
Key Points - Uncommon; Biologically indolent; Found in young women (late 20's)
Solid-Pseudopapillary neoplasms are uncommon, biologically indolent neoplasms. They are composed of discohesive polygonal cells that surround delicate blood vessels. They frequently show cystic degeneration and intracystic hemorrhage. They are often found in young women, median age of 27. Other names that have been given to this neoplasm are: solid and papillary tumor, papillary cystic tumor, solid-cystic tumor, and solid, cystic, and papillary epithelial neoplasm.
| Patient gender | Location in Pancreas | Communication with ducts | Cyst contents | Epithelial lining | Stromal character |
|---|---|---|---|---|---|
| Women | Body/Head/Tail | None | Necrotic/hemorrhagic | Uniform cells with nuclear grooves | Some hyalinization |
Clinical ▼
Symptoms - Nausea; Abdominal pain or fullness; Vomiting
Patients do not often present with SPN-specific symptoms. The symptoms they do present with are often a result of abdominal organs being compressed by large SPN's. Symptoms include: nausea, vomiting and abdominal pain or fullness. In some cases, the cyst may have grown large enough to become a palpable mass found during routine examination. In fact, since symptoms of SPN's are few and non-specific, cysts are often found during routine physical or pelvic examination.
Gross ▼
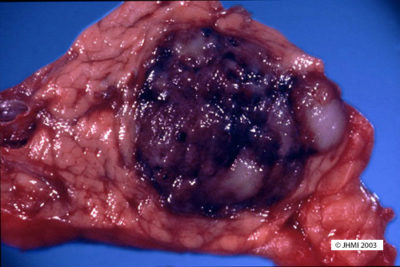
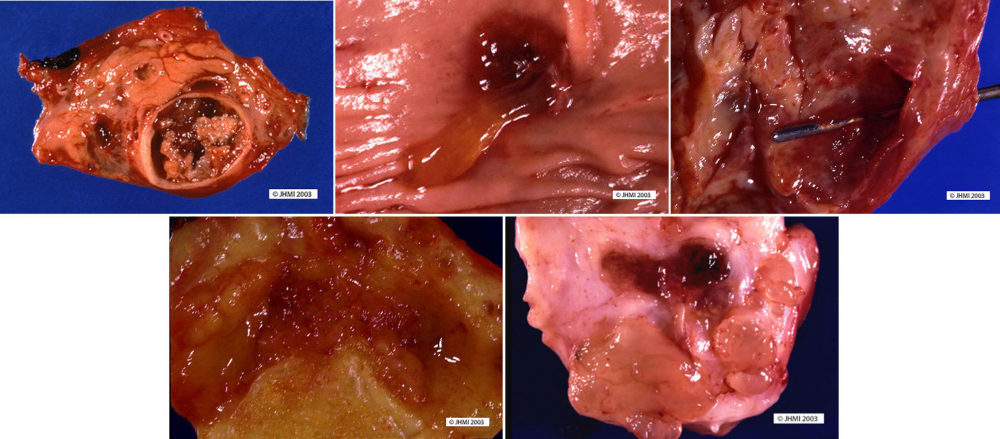
Key Points - Tan to red; Pseudocapsule composed of fibrotic tissue; Cystic degeneration
SPN's may be from 1.5 cm in size to 30 cm, with a mean of 10.5 cm. Small SPN's have a slightly different gross appearance than larger SPN's, smaller SPN's show less cystic change, are soft, tan to red tumors, and have varying degrees of fibrosis. In contrast, larger SPN's have a pseudocapsule composed of fibrotic tissue. Large areas of hemorrhage and cystic degeneration are often present, with ragged fronds of friable tan tumor clinging to edges of cystic cavities. However, there have been examples of SPN's that are completely solid and others that have been entirely cystic.
Histological ▼
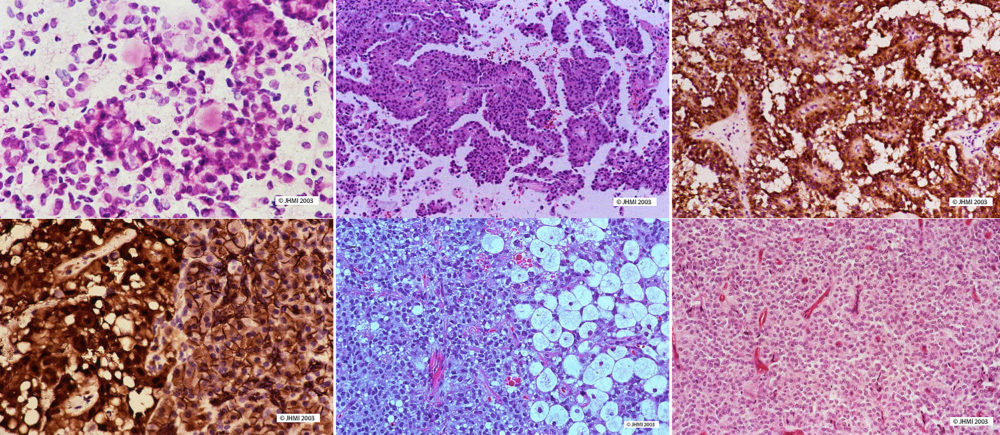
Key Points - Pseudopapillary areas; Hyalin globules
Solid-pseudopapillary neoplasms vary in their appearance depending upon the area of the tumor. For example, in solid areas, sheets and nests of uniform, polygonal epithelioid cells are separated by small vessels, each of which exhibits a variable degree of perivascular collagen. In areas where early degenerative changes are more apparent, the tumor cells situated away from the small vessels begin to drop out, resulting in a residual rim of cells clustered around the small vessels; thus, each perivascular cluster of cells represents a pseudopapilla. Furthermore, there are frequently other manifestations of degenerative changes including aggregates of foamy histiocytes, cholesterol clefts, and hemorrhage. Other histological characteristics of solid-pseudopapillary cysts are hyaline cytoplasmic globules, and round to oval nuclei that exhibit nuclear grooves and convolutions.
Radiological ▼
Key Points
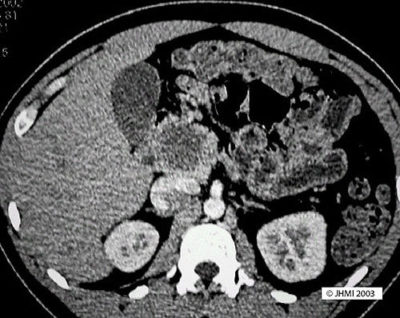
CT, MRI - Sharply circumscribed hypodense lesions
On CT scans, solid-pseudopapillary neoplasms appear as sharply circumscribed hypodense lesions. The cysts have fluid-debris levels and peritubular capsules. SPNs, if small, show no cystic change and appear as pancreatic endocrine neoplasms; larger SPN's will show cystic areas as well as solid areas. On MRI, SPNs are sharply demarcated and have areas of high signal intensity corresponding to foci of hemorrhage on T1-weighted images.
Differential Diagnosis ▼
Key Points - Pseudocysts
The differential diagnosis of solid-pseudopapillary neoplasms includes pseudocysts and other pancreas cysts. However, histological features like pseudopapillary areas and microvasculature should clearly differentiate it from other pancreas cysts. The unique feature of a solid-pseudopapillary neoplasm is the tumor cell, containing foamy cytoplasm, mesenchymal, endocrine, and epithelial differentiation.
In difficult cases, immunostaining for CD 10 and beta-catenin can help establish diagnosis.
References ▼
Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002 Apr; 160 (4); 1361-9.
Intraductal Papillary Mucinous Neoplasms
Definition and Helpful Hints ▼
Key Points - Secrete mucin; Communicate with ducts
Intraductal Papillary Mucinous Neoplasms form papillary projections, secrete mucin, and are intraductal. They lead to ductal dilatation and may produce so much mucin that mucin can be seen oozing from the ampulla of Vater.
| Patient gender | Location in Pancreas | Communication with ducts | Cyst contents | Epithelial lining | Stromal character |
|---|---|---|---|---|---|
| Male | Head | Yes | Mucoid | Pseudostratified tall, columnar | Fibrosis and atrophy |
Clinical ▼
Symptoms - Jaundice; Abdominal pain; Weight loss
IPMN's are usually found in older individuals in their 70's-80's. Some patients may experience symptoms that include: abdominal pain, light stool, weight loss, jaundice, diabetes, and chronic pancreatitis. There may be some hereditary link predisposing some individuals to IPMN's because there has been a documented a correlation with the diagnosis of IPMN's and the presence of malignancies in other organs.
Gross ▼
Key Points - Ductal communication; Located in the head of the organ; Mucin; Papillary projections
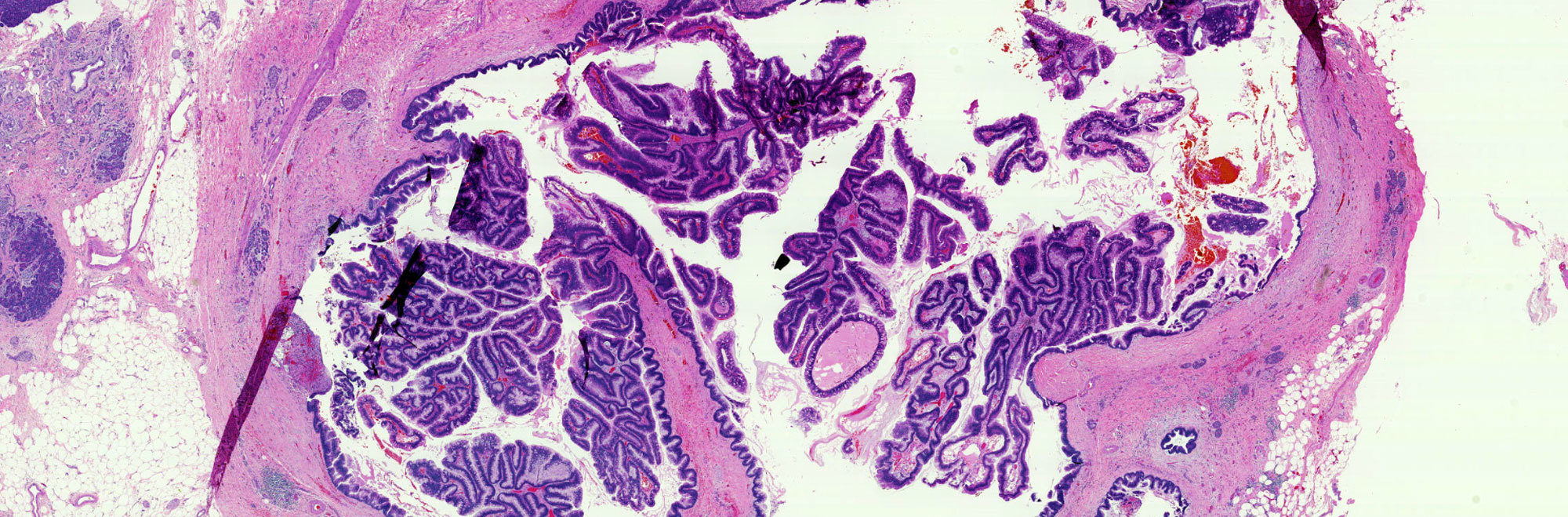
There are three gross features for IPMN's. 1). They involve the pancreatic duct system. 2). They produce copious amounts of extracellular mucin. 3). They often form papillary projections. Therefore, during macroscopic investigation of IPMN's, it is important to note the connection of cysts to the pancreatic duct system. Location of IPMN's within varies. They may be located anywhere in the pancreas, although they are most likely to be found in the head of the organ and involving the main pancreatic duct. Invasive carcinoma is present in 35% of cases, making sampling the specimen an important step of the macroscopic examination of IPMN's.
Histological ▼
Key Points - Two types of papillary projections; Intestinal and Pancreatobiliary
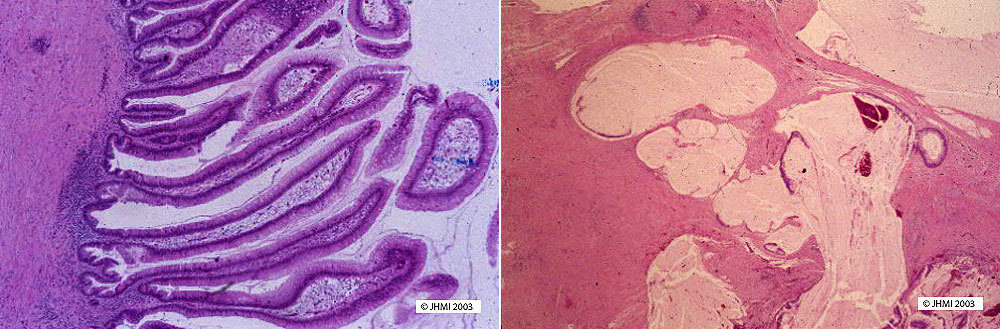
IPMN's may appear similar to mucinous cystic tumors. However, IPMN's involve the ducts of the pancreas and exhibit no ovarian stroma-- two characteristics that distinguish them from mucinous cystic neoplasms.
Papillary projections associated with IPMN's appear in two types of patterns: intestinal type papillae, lined by a border of pseudostratified, tall columnar cells with occasional Goblet cells, and pancreatobiliary type papillae, seen as complex branches with cuboidal cells and large nucleoli. Also seen in IPMN's are paneth cells and neuroendocrine cells.
IPMN's are graded based on the level of cellular atypia they exhibit. Adenomas are considered to have simple mucinous columnar cells, low nucleus-to-cytoplasm ratio, apical cytoplasmic mucin, diffuse and fine chromatin pattern, and no cytological atypia. Borderline cases exhibit pseudostratified pencil-shaped cells, a lack of fusion of micropapillae, and cells with mild to moderate atypia. Carcinoma in situ is marked by severe cellular atypia and fusion of papillae.
When an invasive carcinoma is present, it is often an invasive colloid carcinoma.
Radiological ▼
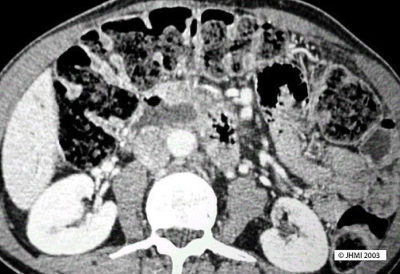
Key Points - Communication with ducts; Endoscopy; Mucin oozing ampulla of Vater
CT scans will typically reveal a cystic mass in the head of the pancreas. The cysts will appear to communicate with the pancreatic duct system.
On endoscopy, mucin can be seen oozing from the ampulla of Vater. ERCP can be used to confirm that the cysts communicate with the pancreatic ducts.
Differential Diagnosis ▼
Key Points - Mucinous Cystic Neoplasms
Mucinous cystic neoplasms are the main entity to consider in the differential diagnosis of IPMN's.Two morphological features distinguish IPMN's from mucinous cystic neoplasms: IPMN's communicate with ducts, mucinous cysts do not, IPMN's also lack an ovarian stroma that is present in mucinous cysts. In addition, mucinous cysts are usually seen in the tail of the pancreas and occur in middle-aged women, while IPMN's are found in the head of the pancreas and occur in older individuals of either sex.