Past announcements, events, and research news.
2024 ▼
10th Annual International Think Tank
The 2024 Sol Goldman Pancreatic Cancer Center International Think Tank on Artificial Intelligence was a wonderful success. The think tank was organized and led by Laura Wood. The over 800 people who registered heard from leading experts from around the world, including Juan Lavista Ferres, Soren Brunak, Jerome Cros, Elana Fertig, Elliot Fishman, and Ashley Kiemen. Also joining as discussants were Lodewijk Brosens, Linda Chu, Alexander Damanakis, James Eshleman, Michael Goggins, Benjamin Haibe-Kains, Jin He, Ralph Hruban, Alison Klein, Jae Lee, Claudio Luchini, Chen Mayer, Shmuel Meitar, Nickolas Papadopoulos, Genevieve Stein-O’Brien, Bert Vogelstein, and Denis Wirtz. The talks were “informative and inspiring,” and the potential power of artificial intelligence in transforming pancreatic cnacer research and patient care was palpable.
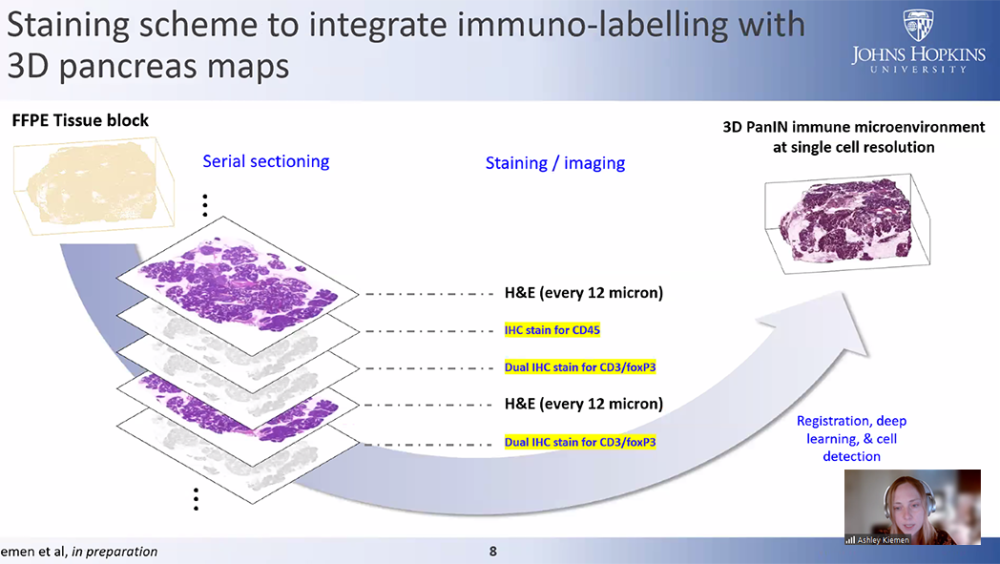
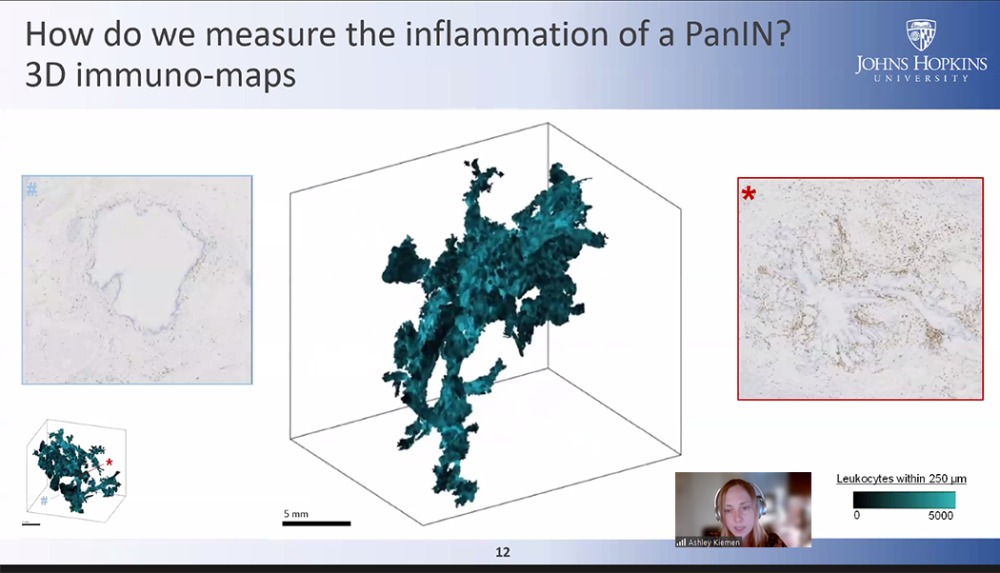
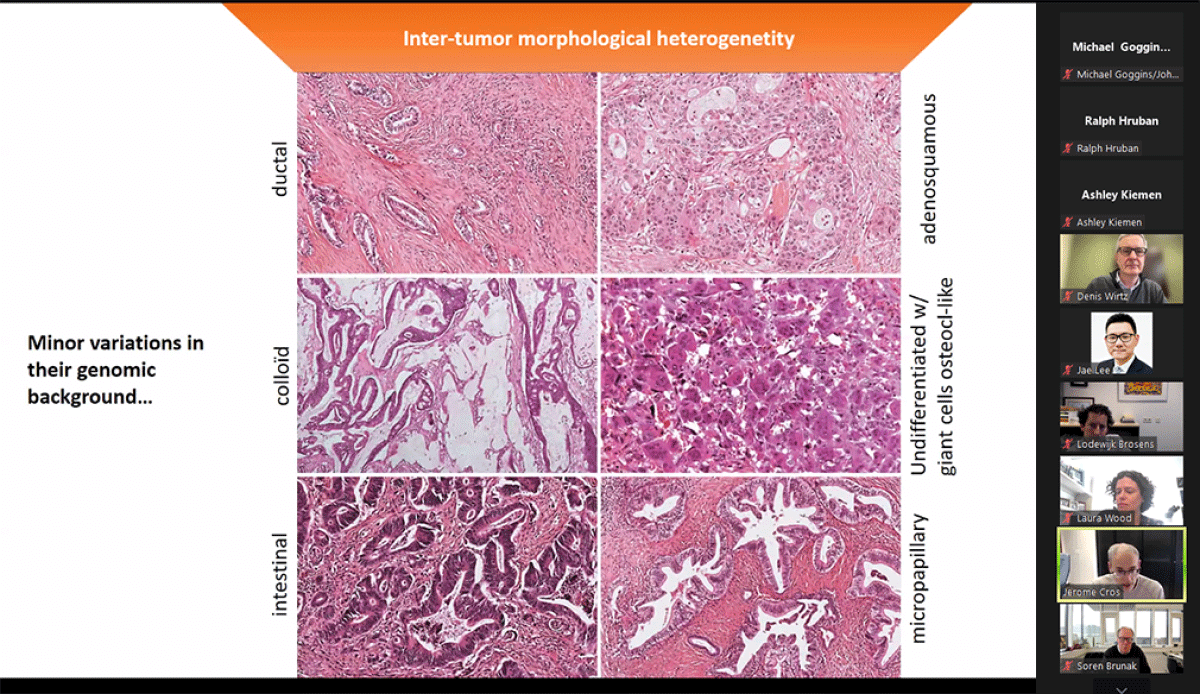

Think Tank 2024
Artificial Intelligence and Pancreatic Cancer
The 10th Annual Sol Goldman Pancreatic Cancer Research Center Think Tank
Hosted by Ralph Hruban, M.D., and Laura Wood, M.D., Ph.D.
April 3, 2024
08:45 AM - 12:00 PM EST
Speakers
Juan Lavista Ferres
Corporate Vice President and Chief Data Scientist
AI for Good Lab at Microsoft
Soren Brunak
Professor of Disease Systems Biology
University of Copenhagen
Jerome Cros
Professor of Pathology
Beaujon Hospital, Université Paris Cité
Elana Fertig
Professor of Oncology
Division Director for Oncology Quantitative Sciences
Johns Hopkins University School of Medicine
Elliot Fishman
Professor of Radiology and Radiological Sciences
Johns Hopkins University School of Medicine
Ashley Kiemen
Assistant Professor of Pathology
Johns Hopkins University School of Medicine
2023 ▼
Cigarette Smoking
Quitting smoking shown to decrease the risk of pancreatic cancer!
Cigarette smoking is one of the leading preventable causes of pancreatic cancer. In most studies, smoking cigarettes doubles one’s risk of developing pancreatic cancer. Veronica Setiawan and colleagues from the University of Southern California just completed a study of cigarette smoking, and the good news is that they found that quitting smoking can reduce the risk of developing pancreatic cancer ! In an analysis of over 180,000 people reported in the journal Cancer Causes and Control (see Excess pancreatic cancer risk due to smoking and modifying effect of quitting smoking: The Multiethnic Cohort Study | Cancer Causes & Control (springer.com) and below), they found that people who smoked more than 50 pack-years (a pack year is calculated by multiplying the number of packs of cigarettes smoked per day times the number of years a person has smoked) had an almost doubled risk of pancreatic cancer. Importantly, they found that every year a person quit smoking corresponded to a 9% decreased excess risk of developing pancreatic cancer! This was especially true for people who quit before the age of 65.
The message is simple- Don’t smoke, and if you do, quit smoking! It may save your life!
Reference:
Bogumil D, Stram D, Preston DL, Pandol SJ, Wu AH, McKean-Cowdin R, Conti DV, Setiawan VW. Excess pancreatic cancer risk due to smoking and modifying effect of quitting smoking: The Multiethnic Cohort Study. Cancer Causes Control. 2023 Nov 4. doi: 10.1007/s10552-023-01811-x. Epub ahead of print. PMID: 37924460.

2023 Think Tank A Success!
The 9th Annual Sol Goldman Pancreatic Cancer Research Center International Think Tank was held on May 24th, 2023. Experts from around the world gathered virtually to discuss the stroma in pancreatic cancer. The meeting was hosted by Laura Wood, M.D., Ph.D.
The speakers and session moderators provided cutting edge insight into the cells that make up the unique stroma around pancreatic cancer cells. Several suggested ways that this unique stroma can be targeted therapeutically.
To give you a flavor of the meeting, listed below are the speakers and the titles of their talks:

Neil Theise, M.D.
Professor of Pathology
New York University Grossman School of Medicine
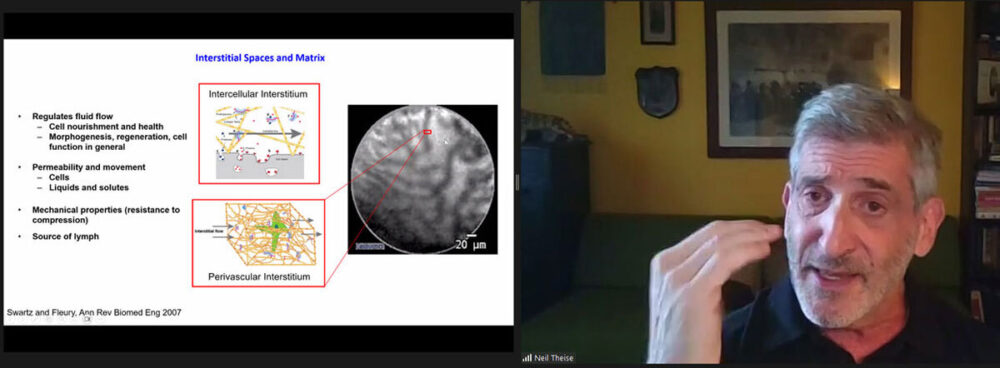
Talk Title: “Body-wide continuity of the human interstitium with focus on the pancreas.”

Ashley Kiemen, Ph.D.
Assistant Professor of Pathology and Oncology
Johns Hopkins University School of Medicine
Talk Title: “Pancreatic cancer cells hijack pre-existing stromal highways to migrate far from the tumor”

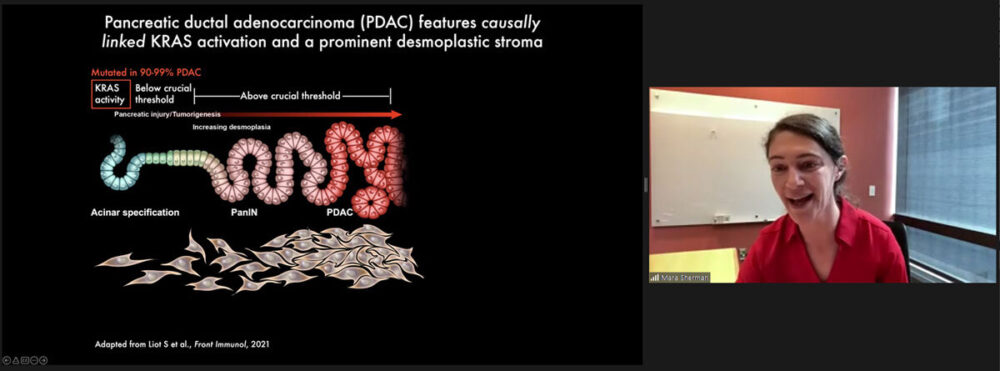
Mara Sherman, Ph.D.
Associate Member, Cancer Biology & Genetics Program
Memorial Sloan Kettering Cancer Center
Talk Title: “Mechanisms and consequences of pancreatic cancer stromal evolution”

Daniel Zabransky, M.D.
Medical Oncology Fellow
Johns Hopkins University School of Medicine
Talk Title: “Aged pancreatic fibroblasts drive pancreatic cancer progression”

Edna (“Eti”) Cukierman, Ph.D.
Professor and Co-Leader of the Cancer Signaling and Microenvironment Program
Co-Director of the Marvin & Concetta Greenberg Pancreatic Cancer Institute
Director of the Spatial Immuno-Proteomics Initiative (as Co-Director of the Histopathology Facility)
Fox Chase Cancer Center / Temple Health
Talk Title: “Clinically meaningful stroma normalization & monitoring in pancreatic cancer”

Sunil Hingorani, M.D., Ph.D.
Professor and Director of the Pancreatic Cancer Center of Excellence
Nancy Armitage Presidential Chair in Pancreatic Cancer
Professor of Medicine, Division of Hematology/Oncology
Director, Pancreatic Cancer Center of Excellence
Fred & Pamela Buffet Cancer Center
University of Nebraska Medical Center
Talk Title: “Targeting the stroma for chemoprevention of mucinous cystic neoplasms”
Some screenshots below:



PanCAN Visit
On Sunday, May 21st, members of PanCAN Washington D.C./Baltimore visited the pancreatic cancer team at Johns Hopkins to learn about our clinical, research and educational efforts. Visitors from PanCAN had the opportunity to meet with members of the Hopkins team in small groups. This allowed for patients and their families to learn about the latest advances, and, conversely, the Hopkins team had the opportunity to learn about the needs of those most impacted by the disease. A special thanks to Anna Somers from PanCAN, and Cordelia Lee from Hopkins, for all of their hard work organizing this wonderful event!











Personalized Vaccine to Treat Pancreatic Cancer
A remarkable study was recently published by Rojas and colleagues in the journal Nature. In their study, sixteen people underwent surgery for pancreatic cancer. The removed cancer was then analyzed, and a personal mRNA vaccine was developed to target 20 unique features (neoantigens) of each of the sixteen cancers. Each participant was then vaccinated with their personal vaccine, and received chemotherapy and an immune check point inhibitor. The results were remarkable. The authors showed that some patients developed an immune reaction (a T cell response) to the unique features present on their cancers. Although half of the patients did not respond, those that did had no evidence of disease 18 months after surgery.
The generation of personalized therapies can be extremely expensive, and this approach has a long way to go (Phase 2 and phase 3 trials are needed), this study “established the feasibility of using mRNA-based neoantigen vaccines for pancreatic cancer.”
Read More

Congratulations, Dr. Ashley Kiemen!
Ashley Kiemen received the Lustgarten Foundation-AACR Career Development Award for Pancreatic Cancer Research in honor of Ruth Bader Ginsburg. This award honors the life and legacy of Justice Ginsburg, who worked tirelessly to advance gender equality, even while battling pancreatic cancer. Congratulations Dr. Kiemen!

Impact of Palliative Care
It can be a difficult subject, but palliative care actually can improve the quality of life for patients with pancreatic cancer. Palliative care is an approach to caring for patients with a serious disease, such as pancreatic cancer, that addresses the person as a whole, focusing on quality of life (see Palliative Care in Cancer - NCI).
Palliative care can be given with or without curative care. Christina Kim and colleagues, from the CancerCare Manitoba Research Institute, recently published their results of a study of patients with advanced pancreatic cancer. In their article in the journal Supportive Care in Cancer (volume 31, page 250) , they report palliative care can improve the quality of life and symptom burden for patients with pancreatic cancer. From these and other results, it is clear that patients with pancreatic cancer should seek early consultation with a palliative care expert.

Think Tank 2023 - The Stroma in Pancreatic Cancer
The 9th Annual Sol Goldman Pancreatic Cancer Research Center Think Tank
Hosted by Ralph Hruban, M.D., and Laura Wood, M.D., Ph.D.
May 24, 2023
08:30 AM - 12:00 PM EST
SPEAKERS
Neil Theise, M.D.
Professor of Pathology
New York University Grossman School of Medicine
Ashley Kiemen, Ph.D.
Assistant Professor of Pathology and Oncology
Johns Hopkins University School of Medicine
Mara Sherman, Ph.D.
Associate Member, Cancer Biology & Genetics Program
Memorial Sloan Kettering Cancer Center
Daniel Zabransky, M.D.
Medical Oncology Fellow
Johns Hopkins University School of Medicine
Edna (“Eti”) Cukierman, Ph.D.
Professor and Co-Leader of the Cancer Signaling and Microenvironment Program
Co-Director of the Marvin & Concetta Greenberg Pancreatic Cancer Institute
Director of the Spatial Immuno-Proteomics Initiative (as Co-Director of the Histopathology Facility)
Fox Chase Cancer Center / Temple Health
Sunil Hingorani, M.D., Ph.D.
Professor and Director of the Pancreatic Cancer Center of Excellence
Nancy Armitage Presidential Chair in Pancreatic Cancer
Professor of Medicine, Division of Hematology/Oncology
Director, Pancreatic Cancer Center of Excellence
Fred & Pamela Buffet Cancer Center
University of Nebraska Medical Center


New Numbers from the American Cancer Society
The American Cancer Society just released their report on the latest cancer statistics, and the news is good! Although cancer continues to be the #2 cause of death in the United States, the risk of dying from cancer has decreased over the past 29 years. This translates into 3.8 million deaths from cancer were averted over these years. (see https://www.cancer.org/latest-news/facts-and-figures-2023)
Slight improvements were seen in the pancreatic cancer numbers. The American Cancer Society estimates that 64,050 Americans will be diagnosed with pancreatic cancer in 2023, and that 50,550 will die from the disease. Of note, the five-year survival rate for individuals diagnosed with pancreatic cancer in the years 1975-1977 was 3%, while for individuals diagnosed in the years 2012-2018 it rose to 12%! While 12% is still way too low, these numbers do offer hope!
In particular, as we noted in an earlier post, it seems that more pancreatic cancers are being detected earlier (at a low stage), and the outcome is much better the earlier cancers are detected (see Recent Trends in the Incidence and Survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results Analysis - PMC (nih.gov))
We believe that the combination of earlier detection and improved therapies will make a difference, and that that we can do much better than 12% survival at 5 years. Importantly, we should all remember Stephen Jay Gould’s words that “the median is not the message.” (The Median Isn’t the Message | Journal of Ethics | American Medical Association (ama-assn.org)) Many individuals live longer than the median. We should all remain hopeful.
2022 ▼
Early Detection Can Save Lives
Pancreatic cancer is the third leading cause of death from cancer. Because pancreatic cancer is often caught at the advanced stage, the Sol Goldman Pancreatic Cancer Research team is finding ways to detect pancreatic cancer earlier when patients have a much better chance of a successful treatment.

DR. MICHAEL GOGGINS & DR. MARCIA CANTO
The early detection team at Johns Hopkins, led by Drs. Michael Goggins and Marcia Canto, just reported in the Journal of Clinical Oncology remarkable success screening people who are at high risk because of their family history for early, curable, pancreatic cancer. The team screened over 1,400 people using endoscopy (endoscopic ultrasound or EUS) and they showed that early curable pancreatic cancers can be detected before a person becomes symptomatic. The five-year survival rate for patient’s in the study whose cancer was detected on screening was an amazing 75%. With an average overall survival of 10 years. There is hope!

2022 Think Tank a Success!
The 2022 Sol Goldman Pancreatic Cancer Research Center International Think Tank was a wonderful success! The think tank, focused on personalized medicine in the treatment of pancreatic cancer, was chaired by Dr. Laura Wood. The think tank was opened by a broad and thoughtful keynote address from Nilo Azad, M.D. Other speakers included Richard Burkhart, M.D., Shudong Wang, Ph.D., FRSC, Steven Gallinger, M.D., Michael Pishvaian, M.D., Ph.D., and Alexander Drilon, M.D. Even though the conference was held virtually because of the pandemic, the meeting was marked by lively and provocative discussions.




Think Tank 2022 - Personalized Medicine in the Treatment of Pancreatic Cancer
The 2022 Sol Goldman Pancreatic Cancer Research Center Think Tank
Hosted by Ralph Hruban, M.D., and Laura Wood, M.D., Ph.D.
May 18, 2022
08:30 AM - 11:30 AM Eastern Time
Please join us for an exciting and informative think tank on Personalized Medicine in the Treatment of Pancreatic Cancer! The think tank will be held via Zoom Webinar and will include some of the leading experts on this exciting topic. See below for a link to register for this free meeting.

NILO AZAD, M.D.
Professor of Oncology
Johns Hopkins University School of Medicine
Lecture title: “Targeted therapies other than KRAS”

RICHARD BURKHART, M.D.
Associate Professor of Surgery
Johns Hopkins University School of Medicine
Lecture title: “Personalized therapy based on patient derived organoids”

SHUDONG WANG, PH.D., FRSC
Professor of Medicinal Chemistry
University of South Australia
Lecture title: “Targeting the cell cycle (CDK4/6)”

STEVEN GALLINGER, M.D.
Professor of Surgery
University of Toronto
Lecture title: “Identifying susceptibility through somatic sequencing”

MICHAEL PISHVAIAN, M.D., PH.D.
Associate Professor of Oncology
Johns Hopkins University School of Medicine
Lecture title: “Know Your Tumor”

ALEXANDER DRILON, M.D.
Chief, Early Drug Development Service
Memorial Sloan Kettering Cancer Center
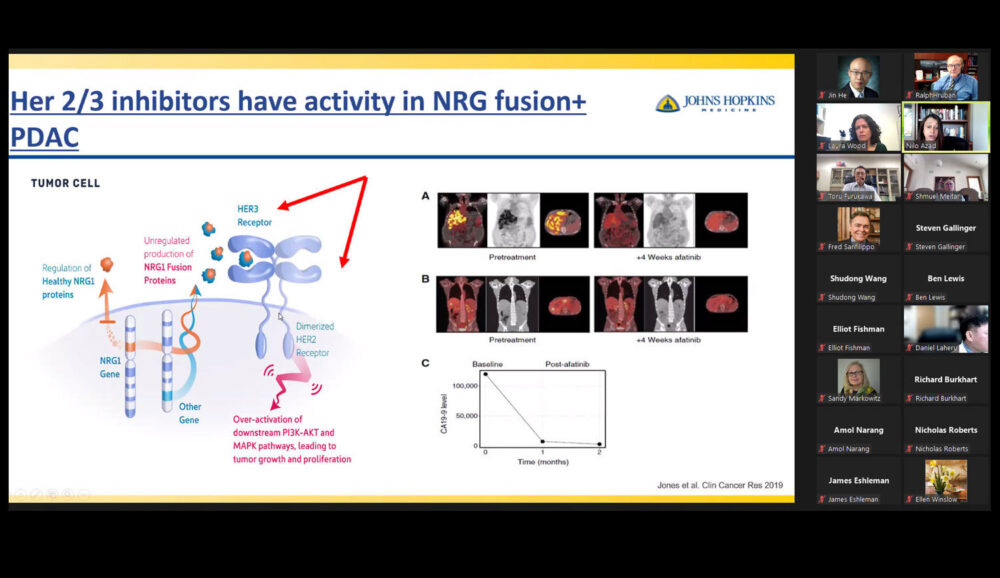
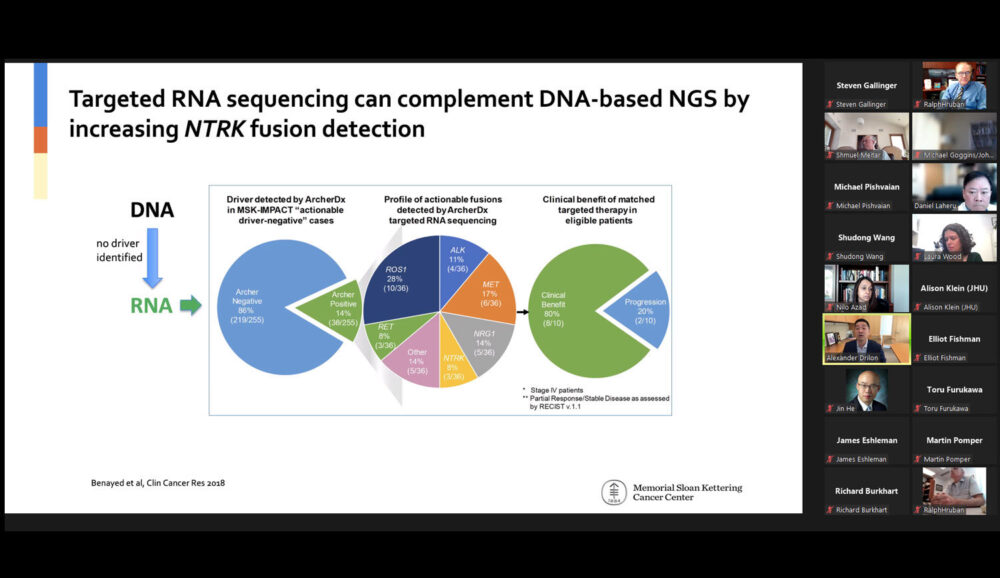
Lecture title: “Targeting NTRK”
2021 ▼
World Experts in Pancreatic Cancer
- November 24, 2021
On World Pancreatic Cancer Day (November 19, 2021), the ExpertScape recognized the World Experts in pancreatic cancer.
Ralph Hruban, M.D., here in the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins, was recognized as the #1 world expert. Michael Goggins, Lei Zheng and Laura Wood, also from Johns Hopkins, were also honored as world experts.
Congratulations to the team at Hopkins!


Hopkins Team Helps Define Protein Expression Patterns in PC
- September 23, 2021
A team of scientists from the Sol Goldman Pancreatic Cancer Research Center published a landmark paper in the journal Cell. The study, the result of an international collaborative effort, compared the proteins made by pancreatic cancer cells to the proteins made by normal cells in the pancreas. In so doing, the team defined the ways in which pancreatic cancer differs from normal. The team used the technology called “mass spectrometry” to characterize the proteins, glycoproteins (proteins with sugar molecules added onto the proteins), and phosphoproteins (proteins with phosphate molecules added). Many thousands of proteins were identified, and the results deposited in publicly available databases for other researchers to use.
Why is this study important? The study is important for three reasons.
- First, many of the known cancer markers are glycoproteins. The discovery of glycoproteins made at high levels therefore identifies potential new pancreatic cancer markers.
- Second, some phosphoproteins are targetable therapeutically. The discovery of phosphoproteins made at high levels in pancreatic cancer cells therefore identifies a number of potential new targets for therapy.
- Third, the database is huge and will be shared freely. It is hoped that scientists will take advantage of it for years to come.
To learn more, visit the Johns Hopkins Newsroom.

Think Tank 2021 - Targeting KRAS
- May 5, 2021
The 2021 Sol Goldman Pancreatic Cancer Research Center Think Tank
Hosted by Bert Vogelstein, M.D. and Ralph Hruban, M.D.
June 16, 2021
08:30 AM – 11:30 AM Eastern Time
Please join us for an exciting and informative think tank on targeting KRAS! The think tank will be held by Zoom and will include some of the leading experts on targeting mutant KRAS. See below for a link to register for this free meeting.

MARIANO BARBACID, PH.D.
AXA-CNIO Professor of Molecular Oncology
Centro Nacional de Investigaciones Oncológicas (CNIO)
Spanish National Cancer Research Center
Lecture title: “In search of Therapeutic Strategies Against KRAS Driven Pancreatic Tumors”

PIRO LITO, M.D., PH.D.
Assistant Member and Attending Physician
Human Oncology & Pathogenesis Program
Memorial Sloan Kettering Cancer Center
Lecture title: “Novel Insights into the Regulation of Mutant KRAS”

YI LIU, PH.D.
CEO, Co-founder
Kumquat Bioscience
Lecture title: “Developing KRAS Inhibitors for Pancreatic Cancer Treatment”

DARRYL B. MCCONNELL, PH.D.
Senior Vice President
Research Site Head Austria
Bei Boehringer Ingelheim RCV GmbH & Co Kg
Lecture title: “The KRAS Drugs en Route”

KEVAN M. SHOKAT, PH.D.
Professor, Department of Cellular & Molecular Chemistry
UCSF Helen Diller Family Comprehensive Cancer Center
Lecture title: “Chemical and Immunological Strategies for Directly Targeting KRAS”

SHIBIN ZHOU, M.D., PH.D.
Associate Professor of Oncology
Sidney Kimmel Comprehensive Cancer Center
Director, Experimental Therapeutics, Ludwig Center for Cancer Genetics
Lecture title: “Redirecting T cells to Kill Cancer Cells with RAS Mutations”


Patients Who Have An IPMN Surgically Resected Need To Be Followed Clinically
- May 4, 2021
Pancreatic cancer is the third leading cause of death from cancer. Because pancreatic cancer is often caught at the advanced stage, the Sol Goldman Pancreatic Cancer Research team is finding ways to detect pancreatic cancer earlier when patients have a much better chance of a successful treatment.
Johns Hopkins’ world-renowned experts, Dr. Anne Marie Lennon and Dr. Marcia Canto, discuss the early detection of pancreatic cancer to talk about cutting-edge diagnostics that will create a different future for those most at risk for pancreatic cancer.


2021 President's Frontier Award Finalist
- February 15, 2021
Congratulations Dr. Laura Wood! Johns Hopkins University named her the 2021 President’s Frontier Award finalist. This award, in recognition of her “Brilliant and Important” pancreatic cancer research, comes with an $80,000 award towards her research.
2020 ▼
Patients Who Have An IPMN Surgically Resected Need To Be Followed Clinically
- November 2020
Patients who have an IPMN surgically resected need to be followed clinically. The team from Hopkins recently reported their experience with close to 450 patients who had an intraductal papillary mucinous neoplasm (IPMN) of the pancreas resected. In the study, reported in the Annals of Surgery (PMID 33201121), the authors clinically followed 449 consecutive patients who had a non-invasive IPMN surgically resected at Johns Hopkins between 1995 and 2018. Remarkably, 16 (3.6%) of the patients developed an invasive cancer in their remnant pancreas during the period of follow-up. The authors estimated the risk of progression was 6.4% at five years after surgery, and 12.9% at 10 years after surgery. These results add to the growing body of evidence that suggest that patients should be clinically followed after they have had surgery for an IPMN because they have a small, but real, risk of developing cancer in their remnant pancreas.

2020 Goldman Think Tank
- October 2020
Due to COVID-19, the Sol Goldman Center held its International Think Tank by Zoom this year!
The focus of this year's Think Tank was on the important topic of pancreatic cysts and precancers. This included "intraductal papillary mucinous neoplasms," and other precancerous lesions of the pancreas. Over 40 scientists participated, including prominent scientists from Japan, The Netherlands, Italy, Israel, and from all across the US.
Dr. Laura Wood gave the keynote, and even though the conference was held by Zoom, the day was filled with lively thought-provoking discussions.



2020 AACR Team Science Prize
- June 2020
The 2020 Team Science Prize from the American Association for Cancer Research was awarded to The Cancer Genome Atlas (TCGA). The TCGA led an effort to sequence the DNA of all major cancer types. Ralph Hruban, M.D. from Johns Hopkins was included as one of the recipients of the AACR's Team Science Prize as he co-led together with Benjamin Raphael, the TCGA's effort to sequence pancreatic cancer. This is an amazing third time that the pancreatic cancer team at Hopkins has been recognized with the Team Science Award. The Hopkins team won it in 2013 for the first sequencing of all of the genes in pancreatic cancer, in 2017 for new approaches to the early detection of cancer (the "liquid biopsy"), and now again in 2020. These awards highlight the collaborative spirit that characterizes the pancreatic cancer research team at Johns Hopkins.
Congratulations to Dr. Hruban and to the team!


Naomi Miller
- April 2020
Singer and comedic actress, Naomi Miller, will be performing a virtual concert on Facebook Live, entitled "Love, Marriage, Children and Liposuction" on Sunday, April 5, 2020 at 10:00 am in memory of her beloved husband, Harvey, who succumbed to pancreatic cancer on January 10, 2020. Naomi is raising support for early detection pancreatic cancer research at Johns Hopkins. Please attend the concert virtually by clicking this facebook link.

Enormous study of the DNA changes across 38 tumor types
- February 2020
The journal Nature just published a series of remarkable papers that integrate a number of large studies of the genetic (DNA) changes in a large number of cancer types. A number of faculty in the Sol Goldman Pancreatic Cancer Research Center participated in these studies. For example, the paper "Pan-cancer analysis of whole genomes," integrated 2,658 whole-cancer genomes (studies of all of the DNA changes) across 38 cancer types, including pancreatic cancer. The investigators used data from the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA), and found that the average cancer has 4 to 5 "driver" mutations (mutations that clearly promote cancer growth), and that "catastrophic" genetic events, called chromothripsis, are more common and occur earlier than previously thought. The driver mutations for pancreatic cancer are usually in the KRAS, TP53, SMAD4 and p16/CDKN2A genes. Large scale studies such as these that integrate multiple datasets can be powerful as they can detect subtle patterns that can be missed in smaller studies. To learn more visit the two links below.

New living cell line of precancer of the pancreas created
- February 2020
Investigators from the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins recently published the first report of a cell line derived from an intraductal tubulopapillary neoplasm (ITPN) of the pancreas. This study, co-lead by principal investigators Dr. Laura Wood and Dr. Nicholas Roberts, reports the molecular and functional characterization of this new cell line.

To create the cell line, the authors used a combination of three-dimensional organoid culture and traditional two-dimensional cell culture. Intriguingly, they demonstrate that the cancer-associated properties of the cell line, such as colony formation and invasion, are intermediate between normal ductal cells and bona fide invasive pancreatic cancer cells, consistent with its identity as a precancerous lesion. This novel cell line represents an important model for further investigation of ITPNs and precancerous pancreatic lesions more generally. Moreover, it highlights three-dimensional culture as a technique to propagate early neoplasms in culture, with the potential to transition to traditional two-dimensional approaches for further studies.


More early stage pancreatic cancers are being detected!
- January 2020
A team led by Dr. Michael Goggins in the Sol Goldman Center just published an extensive review of pancreatic cancers reported in the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) database. They found that the incidence (number) of early stage (stage IA) pancreatic cancers is increasing. Coupled with this increase, they found that the average age of patients with stage I disease is decreasing. They also report that patients with Stage IA disease are more likely to carry insurance (versus Medicaid/none) than are higher-stage cases.
These findings are exciting as it suggests hope for the early detection of pancreatic cancer. Or, as the authors write: "these trends may be the result of improved early diagnosis and early detection."

Risk of Recurrence After Surgical Resection
- January 2020
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are non-invasive precancerous lesions, some of which, over time progress to invasive pancreatic cancer. If an intraductal papillary mucinous neoplasms of the pancreas is removed, it will not progress to invasive cancer. Intraductal papillary mucinous neoplasms of the pancreas therefore represent an opportunity to cure a pancreas tumor, before it becomes cancer.
While removing an intraductal papillary mucinous neoplasm of the pancreas prevents the lesion that was removed from progressing to pancreas, the remnant (remaining) pancreas remains at risk. Just as patients who have had a colon polyp removed need to be more carefully monitored for more colon tumors, so do the patients who have had an intraductal papillary mucinous neoplasms of the pancreas surgically resected need to be monitored for additional pancreas tumors.
Two papers that were just published help answer important questions in the post-operative monitoring of these patients.
First, what is the magnitude of the risk of recurrence after the surgical resection of an intraductal papillary mucinous neoplasm of the pancreas? The reported risk varies, but these two papers, combined with several previously published papers, suggest that the risk of getting a new significant lesion in the remnant pancreas in the five years after surgery is in the range of 5-15%. Patients who had an intraductal papillary mucinous neoplasm of the pancreas with "high-grade dysplasia" resected have a higher risk than do patients who had an intraductal papillary mucinous neoplasm of the pancreas with "low-grade dysplasia" resected.
Second, does the risk ever go down to zero, or do we have to monitor patients indefinitely? Both studies suggest that the risk persists. One cannot identify a certain number of years after surgery where it is safe to stop monitoring. In fact, both studies show that the risk persists well beyond five years after surgery. Why is this important? These studies reinforce a growing body of literature that emphasize the importance of monitoring patients who have had an intraductal papillary mucinous neoplasm of the pancreas resected. They also show that this monitoring should, when clinically indicated, be indefinite.
Visit the Cyst Clinic website2019 ▼
Warm your heart
- December 2019
Warm your heart with an end of the year gift in support of the pancreatic cancer research team at Johns Hopkins! Giving is simple and impactful. Just visit our Support Our Research page. Private giving supports our creative young scientists, private giving helps us pursue new and exciting leads, and most of all, private giving helps us fight pancreatic cancer!
Happy holidays to all, and THANK YOU for your support!

Goldman Center Funds New Grants
- December 2019
We are pleased to announce that the Goldman Center has funded eight exciting new grants. These grants extend from innovative studies of why pancreatic cancer runs in some families, through the genetics of precancerous lesions in the pancreas, to studies of the three dimensional architecture of pancreatic cancer. We look forward to great results from this impactful science!

New 3D Study of Human Pancreatic Cancer
- November 2019
Scientists in the Sol Goldman Pancreatic Cancer Research Center just published a remarkable study of pancreatic cancer using a technique called "tissue clearing." This technique allowed the investigators to study human pancreatic cancers in three dimensions (3D) at the microscopic level. Published in the journal Modern Pathology, this study provides unique insights into how pancreatic cancer invades the small blood vessels (veins) of the pancreas. The invasion of blood vessels allows pancreatic cancer cells to "escape" from the pancreas and spread to other organs such as the liver. The team plans to build on this paper to study the cellular processes that lead to blood vessel invasion. Their hope is to one day block blood vessel invasion.

Global Impact of Pancreatic Cancer
- October 2019
An analysis of the global impact of pancreatic cancer was just published in The Lancet Gastroenterology and Hepatology and the news is sobering. The authors looked at pancreatic cancer in 195 countries over the period of time from 1990 to 2017, and they conclude that "globally, the number of deaths, incident cases, and DALYs (disability adjusted life years) caused by pancreatic cancer has more than doubled from 1990 to 2017." In fact, there were more than 448,000 new cases of pancreatic cancer in 2017. They also predict "a further substantial rise in the absolute number of pancreatic cancer cases" in the coming years. These sobering statistics highlight the need for more research! As the authors conclude, we need to implement better "cost-effective interventions for prevention, early detection, and control of pancreatic cancer."

Screening high-risk individuals for early pancreatic cancer
- October 2019
In the July issue of the British Journal of Surgery Open, an international team, including investigators from the Sol Goldman Center, report the results of screening high-risk individuals for early, curable, pancreatic tumors. The study, a collaboration of 11 surveillance programs, included 76 individuals who had an increased risk of developing pancreatic cancer based on either a strong family history of pancreatic cancer, or because they carried a gene that predisposes to pancreatic cancer (such as BRCA2). The investigators found that older age (>65 years), female sex and carriage of a gene that predisposes to pancreatic cancer were associated with the presence of a high-risk precursor lesion or invasive pancreatic cancer. Why are these findings important? The results will help guide programs for the early detection of pancreatic cancer. Patients who are screened and found to have a lesion in their pancreas may be prioritized for surgery if they are older, female and carry a gene that predisposes to pancreatic cancer.
Another great example of team science fighting pancreatic cancer!


2019 Nobel Prize Winner
- October 2019
Congratulations to Dr. Gregg Semenza- one of the winners of the 2019 Nobel Prize in physiology or medicine! Gregg is on the faculty here at Johns Hopkins, and he was awarded the prize for his discoveries of how cells sense and adapt to changes in oxygen concentrations. This is important for pancreas tumors, because it turns out that one of the tumors that arises in the pancreas, serous cystadenoma, is caused by a mutation in the VHL gene, and VHL is one of the genes that Gregg has shown is important in sensing and responding to changes in cellular oxygen levels. Congratulations Gregg!

New test to guide the management of cysts in the pancreas
- August 2019
Cysts (collections of fluid caused by small tumors) of the pancreas present a real clinical challenge. While most are entirely harmless, some are precancerous and, if left untreated progress over time to pancreatic cancer. The problem is that it can be hard for doctors to know which cysts should be removed surgically and which are safe to follow without surgery. In the July 17th issue of the journal Science Translational Medicine, S. Springer and colleagues in the Sol Goldman Pancreatic Cancer Research Center report a new approach that could help guide the clinical management of patients with a cyst in their pancreas. The test, called "CompCyst" integrates a combination of clinical features, imaging findings, and cyst fluid genetics. Tested on over 400 patients, CompCyst proved to be much more accurate than just the clinician's judgement on whether or not a cyst needs to be removed surgically. The authors concluded that "application of the CompCyst test would have spared surgery in more than half of the patients who underwent unnecessary resection of their cysts. CompCyst therefore has the potential to reduce the patient morbidity and economic costs associated with current standard-of-care pancreatic cyst management practices." This test is not available yet as an approved clinical test, but pathologists in the Goldman Center are working to bring a clinical test to practice.

IPMN precursor lesions are more complex than thought
- July 2019
In the advance on-line June 5th issue of the journal Gastroenterology, Dr. Laura Wood and colleagues describe the results of the most detailed study to date of intraductal papillary mucinous neoplasms (IPMNs). IPMNs are a precancerous lesion of the pancreas. Like polyps of the colon, IPMNs of the pancreas are common and some, if left in place, can progress to invasive cancer over years. Understandings IPMNs is therefore a critical basis for preventing the development of invasive pancreatic cancer. In the study in Gastroenterology, Dr. Wood and colleagues used a panel of cutting edge molecular biology technologies, including whole-exome sequencing and in situ detection of mutations, to characterize IPMNs. They discovered that a single IPMN lesion can actually contain multiple genetically separate tumors (multiple independent clones). They also found that as IPMNs advance towards invasive cancer, a single clone dominates but there is still genetic complexity. This study, from the Sol Goldman Center, fundamentally changes our understanding of how IPMNs arise and progress to cancer. As the authors conclude, "Increasing our understanding of the mechanisms of IPMN" - could lead to strategies to identify patients at increased risk for pancreatic cancer.

Blood test to follow cancer treatment
- June 2019
In the May 29th advance on-line version of the journal Clinical Cancer Research, Drs. Vincent Groot and James Eshleman and their colleagues from the Sol Goldman Pancreatic Cancer Research Center describe a new blood test that can be used to follow effectiveness of treatment for patients with pancreatic cancer. The test for mutated KRAS genes shed by pancreatic cancer cells has been previously shown to work in the research setting. In this study, Dr. Eshleman and colleagues clinically validated the KRAS assay in a Clinical Laboratory Improvement Amendments (CLIA) and College of American Pathology (CAP) certified clinical laboratory. Using this test in the clinical setting, the investigators were able to show that measurement of KRAS in a CLIA laboratory setting can be used to predict recurrence and survival in patients with pancreatic cancer.
This is important because it suggests that the optimal therapy for patients with pancreatic cancer can be guided by their level of KRAS in their blood. Patients whose KRAS levels drop after therapy are likely responding to treatment and can be kept on the same treatment, while patients whose KRAS levels rise after therapy are likely not responding to treatment and their treatment can be changed.

The Importance of BRCA Genes in Guiding Therapy
- June 2019
Some (5-10%) pancreatic cancers are caused by inherited mutations in one of the breast cancer genes (called BRCA1, BRCA2 and PALB2). These inherited gene mutations are important for two reasons. First, they are important for other family members, as other family members may also inherit one of these mutations and they would benefit from knowing that they are at increased risk of developing cancer themselves. Second, pancreatic cancers that arise in patients with an inherited mutation in BRCA1, BRCA2 or PALB2 appear to be particularly sensitive to specific forms of chemotherapy. This second point is highlighted in the recently published POLO trial. The results of this phase III clinical trial were announced at the recent meetings of the American Society of Clinical Oncology. In this trial patients with a known BRCA gene mutation whose disease had not progressed on afirst-line platinum-based chemotherapy were treated with the drug called olaparib, a "PARP-inhibitor." They found that a clinically-meaningful and statistically significant improvement in progression-free survival for patients treated with olaparib compared to controls. The results of this trial are exciting, and highlight the importance of genetic testing for patients with pancreatic cancer. Indeed, the recent NCCN guidelines recommend genetic testing for all patients with pancreatic cancer. To learn more about the genes that cause pancreatic cancer, click here.
To learn more about this trial, see this article from AP News.

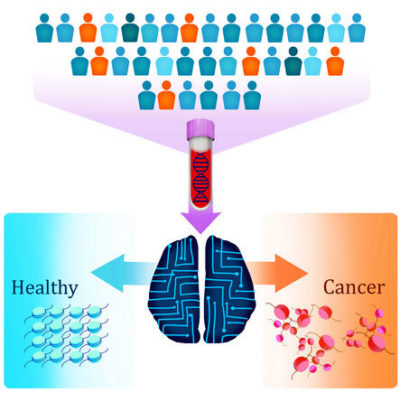
New Blood Test for Cancer
- May 2019
A team of scientists at Johns Hopkins, led by Victor Velculescu, M.D., Ph.D., report a new approach to a blood test for cancer. Reported in the journal Nature, the test can be used to detect seven different types of cancer, including pancreatic cancer. The test, called "DELFI" (DNA evaluation of fragments for early interception) detects abnormal fragments of DNA in the blood that have been shed by cancer cells. This technique takes advantage of the fact that cancers "package" their DNA differently than normal cells, and this unique packaging leads to unique patterns of DNA fragmentation that can be detected in the blood. DELFI detected close to 75% of cancer patients, and, remarkably, when combined with another blood test, DELFI could detect 91% of cancers.

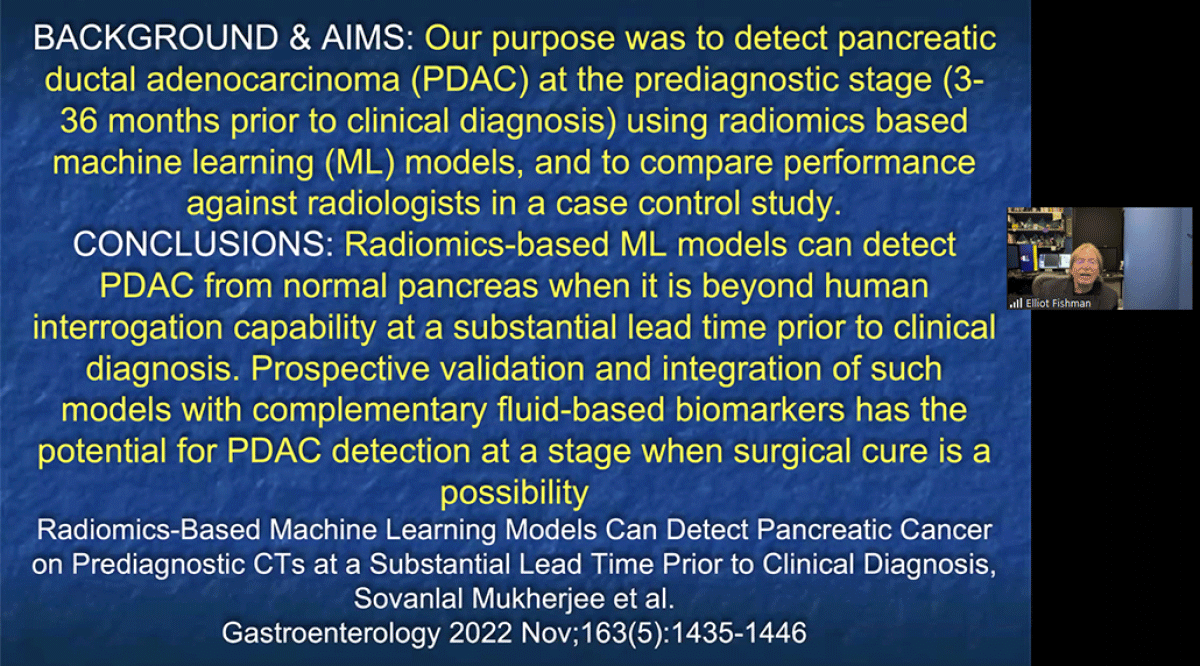
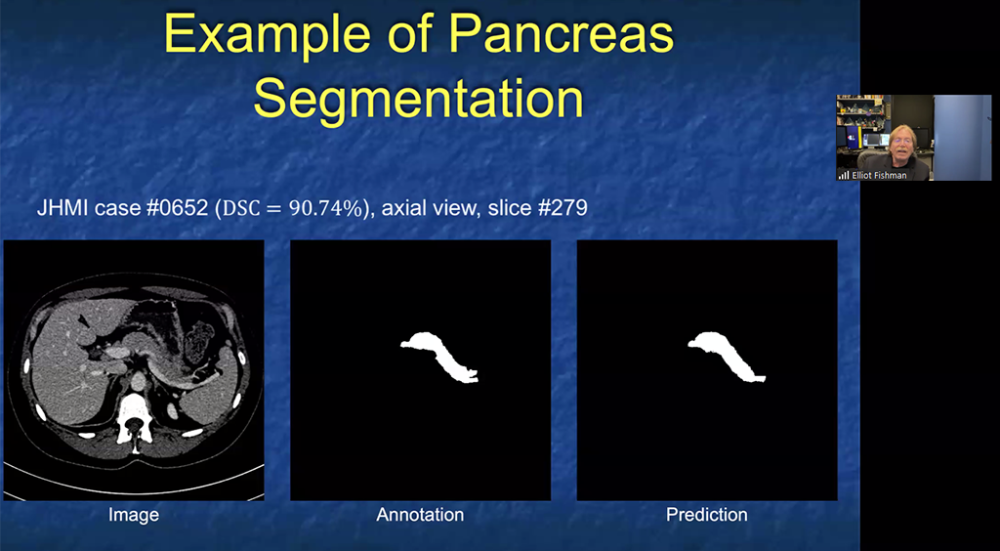
Using computer algorithms to identify pancreatic cancer
- May 2019
Researchers from the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins recently participated in a study that applied the new computer tool called "radiomics" to CT scans of the pancreas. The study, reported in the April issue of the American Journal of Roentgenology (AJR) showed that radiomics features can be used to identify pancreatic cancer in CT scans. The Hopkins team, which included radiologists, pathologists, computer scientists, and cancer biologists, analyzed computed tomography (CT) scans from 380 patients - 190 with pancreatic cancer and 190 healthy controls. Their initial analysis revealed 40 radiomics features which were then used to classify a set of validation scans as either cancer or normal. This new classifier had >99% accuracy and correctly identified all cases of pancreatic cancer, suggesting it could be a powerful complement to human review of imaging data. The study suggests that computer programs can be developed that will help radiologists diagnose pancreatic cancer more accurately. To learn more, visit: https://www.ajronline.org/doi/full/10.2214/AJR.18.20901

4th Sol Goldman International Conference
-April 2019
The 4th Sol Goldman International Conference on Pancreatic Cancer was held April 10-11, 2019 on the Mount Washington campus of the Johns Hopkins University School of Medicine. The focus of this year's Think Tank was familial pancreatic cancer. The discussions were broad and deep, and most of all wonderfully thought provoking. The think tank ended with a lively discussion of impactful ways to move the field forward. Participants included:
Emmanuel Antonarakis, MD (Johns Hopkins University School of Medicine),
Susan Domchek, MD (University of Pennsylvania),
Elliot Fishman, MD (Johns Hopkins University),
Michael Goggins, MD (Johns Hopkins University),
Ralph Hruban, MD (Johns Hopkins University),
Reed Jobs (Emerson Collective),
David Kelsen, MD, PhD (Memorial Sloan Kettering Cancer Center),
Scott Kern, MD (Johns Hopkins University),
Kenneth W. Kinzler, PhD (Johns Hopkins University),
Alison Klein, PhD, MPH (Johns Hopkins University),
Benjamin Lewis, MD (New York-Presbyterian/Columbia),
Prof. Christopher Lord, DPhil (The Institute of Cancer Research, London, United Kingdom),
Nicholas Papadopoulos, PhD (Johns Hopkins University),
Nicholas Roberts, VetMB, PhD (Johns Hopkins University),
Patrick Soon-Shiong, MD (NantWorks),
Ian Tomlinson, PhD, FRCPath, FMedSci (University of Birmingham),
Bert Vogelstein, MD (Johns Hopkins University),
Joshua Vogelstein, PhD (Whiting School of Engineering, Johns Hopkins University),
R. Jacob Vogelstein, PhD (Camden Partners Holdings, LLC),
George Zogopoulos, MD, PhD (McGill University),
Lee Zou, PhD (Harvard Medical School).




Emma's Crafts for a Cure
- January 2019
Emma Shaw is seven years old and lost her grandfather to pancreatic cancer in 2017. To honor his memory she started "Emma's Crafts for a Cure". Emma makes and sells jewelry at local events, specifically to raise money for the Sol Goldman Pancreatic Cancer Research Center. Over her Christmas break Emma and her parents came to Johns Hopkins, toured the pancreatic cancer research labs and presented a check for over $500 to support research in the Goldman Center. What a remarkable young woman!


Remembering John W
- January 2019
On August 18, 2018, a group of John's friends gathered together to remember John and to ride to raise donations in support of Pancreatic Cancer Research at the Johns Hopkins Sol Goldman Pancreatic Cancer Research Center. This was the 7th annual REMEMBERING JOHN (W) ride and the group covered 20+ miles on the York Heritage Railtrail from Hanover Junction to the Mason-Dixon line and back. They raised $3,300 for cancer research.
2018 ▼
Goldman Center Funds Seven Exciting Grants
- December 2018
The Sol Goldman Center will fund seven innovative grants in 2019. These grants, selected based on the novelty and potential impact of the science, are listed below. A GREAT example of the power and profound effect of private giving in the war against pancreatic cancer!
Jody Hooper, M.D.
Associate Professor of Pathology
Evaluating Efficacy of Rapid Autopsy Specimens in Pancreatic Cancer
Alison Klein, M.H.S., Ph.D.
Professor of Oncology and Pathology
Assessing the impact of heritable promoter methylation of Familial Pancreatic Cancer Genes on pancreatic cancer risk
Hai-Quan Mao, Ph.D.
Professor of Materials Science and Engineering and Biomedical Engineering
Nanoparticle platform for lymph node targeted neoantigen-based vaccine therapy for pancreatic cancer
Nicholas Roberts, Vet.M.B., Ph.D.
Assistant Professor of Pathology and Oncology
The role of ATM variants of unknown significance in familial pancreatic cancer
Elizabeth Thompson, M.D., Ph.D.
Assistant Professor of Pathology and Oncology
Site-specific differences in the tumor immune microenvironment and T cell repertoire in metastatic pancreatic adenocarcinoma
Laura Wood, M.D., Ph.D.
Associate Professor in Pathology and Oncology
Identification of the Molecular Drivers of Vascular Invasion in Pancreatic Cancer
Pei-Hsun Wu, Ph.D.
Assistant Research Professor in Chemical and Biomedical Engineering
Quantitative characterization of the immune microenvironment of pancreatic intraepithelial neoplasia (PanIN) in three-dimensions

Local Recurrence of Pancreatic Cancer
- November 2018
In a collaborative study, researchers from the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins and the University of Verona in Italy analyzed the genetic alterations in pancreatic cancers that recurred locally after surgery. The study, published this week in Modern Pathology, showed that half of the cases represented true recurrences - the original pancreatic cancer occurred again in the remaining pancreas. Intriguingly, in two cases, the cancer in the remnant pancreas shared no genetic alterations with the original primary tumor, suggesting that the recurrence was actually a second independent pancreatic cancer. These data shed new light on pancreatic cancer progression after surgery and show that all recurrences after surgery are not the same, suggesting another time point at which clinical care might be better tailored to individual patients.

Top Experts in Pancreatic Cancer
- November 2018
In recognition of Pancreatic Cancer Awareness Month, ExpertScape announced this morning its list of the top experts worldwide in Pancreatic Cancer. Read the press release here.
Johns Hopkins tops the list of the World's Top Institution for Pancreatic Cancer, and two physician-scientists here at Johns Hopkins are listed in the World's Top Experts for Pancreatic Cancer (Dr. Ralph Hruban is #1 and Dr. Christopher Wolfgang as #4). Congratulations to all!

Giving Tuesday
- November 2018
In this season of Thanksgiving, we would like to thank all of the donors whose generosity is so critical for the research carried out in the Sol Goldman Pancreatic Cancer Research Center. Philanthropy at all levels has enabled all of the key discoveries at our center, and it continues to fund novel ideas to improve the diagnosis and treatment of pancreatic cancer. In addition, philanthropic donations fund new investigators so we can develop the next generation of pancreatic cancer researchers. If you would like to donate to the Sol Goldman Pancreatic Cancer Research Center for Giving Tuesday or anytime throughout the year please visit our Supporting Our Research page. Thank you for helping us to fight pancreatic cancer!

Research Study in Wood Laboratory
- November 2018
Researchers in the Sol Goldman Pancreatic Cancer Research Center in the laboratory of Dr. Laura Wood recently reported the first single-cell DNA sequencing study in human pancreatic neoplasms. The study, published online today in the Journal of Pathology, focused on intraductal papillary mucinous neoplasms (IPMNs), and important precursor of pancreatic cancer. The study identified a subset of IPMNs with multiple mutations in the initiating driver gene KRAS in unique tumor clones, suggestive of polyclonal evolution. In addition, they found multiple mutations in later occurring driver genes such as RNF43 and ARID1A localized to unique subclones in individual IPMNs, suggesting convergent evolution of later driver gene mutations. These studies provide new insights into the earliest stages of pancreatic tumorigenesis and suggest that it is a much more complex process than previously appreciated.

Significant Basic Science and Clinical Advances!
- September 2018
Two nice papers on pancreatic cancer were published today. One in Nature by Alvin Makohon-Moore and colleagues and the other in Cancer Discovery by Aguirre and colleagues. The study in Nature highlights basic science advances, and the study in Cancer Discovery clinical advances. In the Nature paper the authors, which include the team from Hopkins, combined genetic sequencing with the mathematics used by evolutionary biologists to show that small precancerous lesions in the pancreas can spread within the ducts (tubes) of the pancreas creating multifocal precancerous lesions, some of which can then progress to invasive cancer. This study is important because it establishes a firmer scientific basis for early detection and prevention of pancreatic cancer.
In the paper in Cancer Discovery, the authors also used genetic sequencing. They analyzed a series of 71 pancreatic cancers and found that two of the cancers had unique genetic mutations (BRAF gene deletions) that made the cancers uniquely susceptible to specific drugs (MAPK pathway inhibitors). This paper adds to the growing body of evidence that suggests that in a small minority of patients sequencing their pancreatic cancer may provide patient-specific therapeutic targets (individualized medicine).

Hope for High-Risk Individuals
- June 2018
In an advance on-line publication in the journal Gastroenterology Dr. Marcia Canto and colleagues in the Sol Goldman Pancreatic Cancer Research Center report on a research study in which they screened and followed 354 people who were at high-risk of developing pancreatic cancer because they carried a gene or because they had a strong family history of pancreatic cancer. 14 of the people screened developed pancreatic cancer in the 16 years of the study. 9 of the ten who had their cancer detected by screening had surgically resectable pancreatic cancer, and 85% of these patients with pancreatic cancer were alive at three years. These findings provide hope for the early detection of pancreatic cancer in high-risk individuals. Dr. Canto and her team have helped form an international consortium to establish best practices for high-risk individuals across the world.

Think Tank
- April 2018
The 2018 Sol Goldman Pancreatic Cancer Think Tank was a wonderful success! The think tank, which focused on artificial intelligence, brought together the top scientists, mathematicians and experts in pancreatic cancer from around the world. Great people sharing new ideas and new approaches. The way science should be! Wonderfully exciting!






Art Creates Cures
- March 2018
Inspired by his own battle with pancreatic cancer, art entrepreneur Budi Tek was moved to establish the Art Creates Cures Foundation (ACC) to give those diagnosed with this deadly disease every possible chance. Joining together with partners Sotheby's, and Johns Hopkins Medicine, the goal of ACC is to raise funds to support the development of an innovative "early detection test" as well as a cure for pancreatic cancer. ACC's goal is to support research that advances the understanding of the biology of the disease, translates this new knowledge into better patient care, and in so doing, improves the lives of patients living with pancreatic cancer.
UPCOMING EVENTS
To kick off fundraising for this worthy cause, Art Creates Cures will present the premier annual Art Creates Cures Charity Gala and Auction on Wednesday, 28 March, 2018 to launch the foundation. The inaugural event of the newly established non-profit foundation will take place at the elegant Four Seasons Hotel in Hong Kong. Bringing together leaders in the art and science communities, the foundation aims to accelerate and transform cancer research by uniting the creativity and ingenuity of artists and that of biomedical scientists. The gala will allow guests to support pancreatic cancer research through their financial contributions as well as give them the opportunity to bid on unique experiences. Contemporary artworks donated by collectors and such world-renowned artists as Zhang Huan, Hu Junjun, Xu Bing, Zhao Bandi, Yang Fudong, among others, will be sold as part of the Sotheby's 2018 Spring Sale with the proceeds also supporting Johns Hopkins' fight against pancreatic cancer.
For more information about ACC, please contact Justine Alexandria at [email protected] or visit https://artcreatescures.org.

2017 ▼
100 grants funded!
- December 2017
The Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins has reached a remarkable milestone! The Goldman Center just funded its 100th pancreatic cancer research grant! Beginning 2005, income from The Sol Goldman Pancreatic Cancer Research Center endowment has funded 100 grant proposals, benefiting 41 principal investigators from 16 departments exploring novel avenues of research. What remarkable impact!

Congratulations, Dr. Ralph Hruban!
- November 2017
Dr. Ralph Hruban, Director of the Sol Goldman Pancreatic Cancer Research Center was recently recognized by Clarivate Analytics as one of the world's most highly cited researchers (https://hub.jhu.edu/2017/11/16/clarivate-highly-cited-researchers-2017). This recognition is based on the number of citations for papers published between 2005 and 2015. Citations are when one researcher references another researcher's paper in their work. A wonderful indicator of the impact of the Goldman Center!

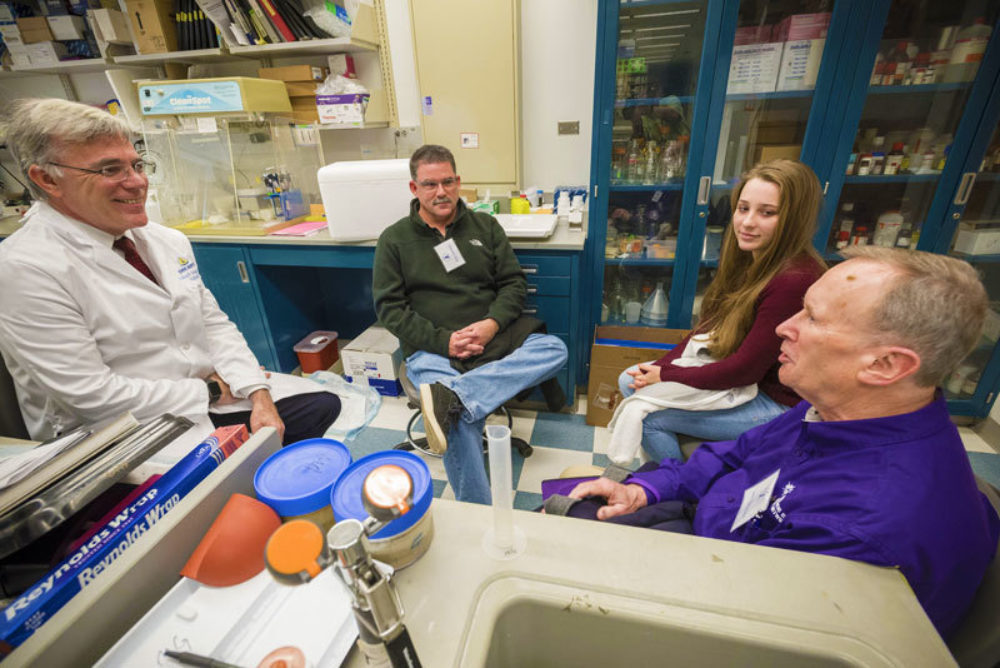
PanCAN Visit
- November 2017
The Baltimore and Washington D.C. affiliates of PanCAN, the national pancreatic cancer advocacy group, visited the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins on Sunday, November 12, 2017. The morning started with Dr. Ralph Hruban inviting the patients, family members, advocates and the Hopkins team to introduce themselves and share their connection to pancreatic cancer. The visitors then broke up into eight groups for small informal meetings with the doctors, scientists and health care providers at Hopkins. Each group spent 10-15 minutes with a member of the Hopkins team, and then moved to the next team member. Dr. Hruban described it as "speed dating!" Most importantly, the PanCAN visitors got a close-up and personal tour of the labs and the opportunity to meet and chat with a number of the world leaders in pancreatic cancer care and research. The team members from Hopkins who participated in the event included:
- Dr. Lei Zheng: chemotherapy for pancreatic cancer
- Dr. James Eshleman: New approaches to discovering novel therapies
- Dr. Elliot Fishman: Recent advances in radiology
- Dr. Michael Goggins: Early detection of pancreatic cancer
- Dr. Jin He: minimal invasive surgery in the management of pancreatic cancer
- Dr. Jody Hooper: Rapid Autopsy program
- Dr. Alison Klein, Nancy Porter & Sharon Varghese: Familial pancreatic cancer – The NFPTR
- Dr. Nick Papadopoulos: Genetic discoveries









TCGA New Publication
- August 2017
The Cancer Genome Atlas (TCGA) research network (the US government's effort to sequence the DNA in all of the major types of cancer), just published their results from a complete genetic analysis of 150 pancreatic cancers! In the August 14th issue of the journal CancerCell, the TCGA authors describe a detailed integrated (DNA, RNA and protein) analysis of the 150 pancreatic cancers. The major genes driving pancreatic cancer are described, and the authors identified two main subtypes of pancreatic cancer (basal-like/squamous and classical/progenitor). While this study does not have direct patient impact yet, it does represent a major milestone in our understanding of this disease. Special congratulations to the many scientists from Johns Hopkins who contributed to this study!
Cory Sandone and Carolyn Hruban created this medical illustration on the right in honor of the study. The Cancer Genome Atlas (TCGA) Research Network performed integrated genomic, transcriptomic and proteomic profiling of 150 pancreatic ductal adenocarcinomas (PDACs). PDACs (depicted as a star-shaped mass in the pancreas) are composed of a few neoplastic cells (marbles labeled with crabs) admixed with greater numbers of non-neoplastic cells (white marbles). The team employed a novel analytical strategy (depicted as the sieve) to identify neoplastic cell-specific molecular subgroups (depicted as the buckets of enriched populations of marbles with crabs).


Sol Goldman Professorship
- June 2017
A wonderful ceremony was held on Monday June 19th, establishing the Sol Goldman Professorship in Pancreatic Cancer Research in the Department of Pathology. This professorship honors the legacy of Sol Goldman and provides critical stability and flexibility to our faculty so that they can take advantage of important opportunities for innovative research. Michael Goggins, a world leader in the early detection of pancreatic cancer and long-term member of the Sol Goldman Pancreatic Cancer Research Center, was inducted as the first recipient of the Goldman Professorship. Congratulations Mike!

Pancreatic cancer patients may live longer by traveling to academic hospital for operation
- May 2017
A recent paper published in the Journal of the American College of Surgeons reports that traveling to an academic medical center such as Johns Hopkins for surgical resection of a pancreatic cancer is associated with higher quality surgical care. Although they found better care at high-volume surgical centers, they report that few patients travel for their cancer operations. To learn more, visit:
https://www.sciencedaily.com/releases/2017/05/170501131824.htm or https://www.sciencedirect.com/science/article/pii/S1072751517302685


Congratulations Dr. Weiss!
- April 2017
Johns Hopkins pancreas surgeon Dr. Matthew Weiss was inducted into the Miller-Coulson Academy as a Miller-Coulson Scholar. This high honor recognizes clinical excellence- "The clinically excellent academic physician who has achieved a level of mastery in communication & interpersonal skills, professionalism & humanism, and negotiation of the healthcare system. Such physicians are exemplary with respect to diagnostic acumen, knowledge, and their scholarly approach to clinical practice. They exhibit a passion for patient care, and they explicitly model all of the above to medical trainees, earning them a reputation for being exceptional." Congratulations Dr. Weiss!
2016 ▼
Hypermutation in Pancreatic Cancer
-November 2016
Scientists in the Sol Goldman Pancreatic Cancer Research Center at Hopkins collaborated on an international study that was recently published in the journal Gastroenterology. The authors sequenced 385 pancreatic cancers and identified a small group of tumors that had many many more mutations (DNA changes) than did the other cancers. These "hypermutation" cancers are important to recognize because they may be more responsive to treatment with immunotherapies. Pubmed Abstract link »

Johns Hopkins Scientists Track Metabolic Pathways to Find Drug Combinations for Pancreatic Cancer
- September 2016
Cancer researchers have long observed the value of treating patients with combinations of anti-cancer drugs that work better than single drug treatments. Now, in a new study using laboratory-grown cells and mice, Johns Hopkins scientists report that a method they used to track metabolic pathways heavily favored by cancer cells provides scientific evidence for combining anti-cancer drugs, including one in a nanoparticle format developed at Johns Hopkins, that specifically target those pathways.
"We have to hit cancer cells from more than one angle, and that's made it important to learn how to combine drugs that hit the right combination of pathways," says Anne Le, M.D., H.D.R., assistant professor of pathology at the Johns Hopkins University School of Medicine and member of the Johns Hopkins Kimmel Cancer Center.
Le says that the study of so-called metabolomics to track biochemical reactions in cancer and other cells should help scientists decide how best to combine drugs. A report of the scientists’ work will appear online the week of Aug. 22 in Proceedings of the National Academy of Sciences.
For the study, Le and her collaborators at Johns Hopkins, including Barbara Slusher, Ph.D., an expert in drug discovery, and Justin Hanes, Ph.D., a nanomedicine expert, started with an experimental drug called BPTES and injected it in mice with implanted human pancreatic tumors. BPTES has been used in animal models for a variety of cancers but has not substantially reduced tumor sizes, probably because the drug concentration in tumor tissue is not high enough when using conventional drug formulation methods, say the scientists.
With this in mind, scientists from the Center for Nanomedicine at Johns Hopkins, led by Hanes, encapsulated the BPTES in a nanoparticle capsule coated in polyethylene glycol, a molecule used widely in medicines and industrial products, using a method they developed to provide a more uniform coating. The nanoparticle, according to the scientists, helps the drug slip through capillaries near cancer cells and remain within the tumor longer than it would otherwise.
After 16 days, eight mice treated with encapsulated BPTES had tumors half the size of another eight mice treated with nanoparticles containing no drug. BPTES not encased in the nanoparticle delivery system had little effect on tumor size in 12 human tumor-bearing mice. "This shows that the nanoparticle-encapsulated drug is more effective in tumor reduction than the drug alone in these animal models," says Le.
But their overriding interest in BPTES, says Slusher, was in how it works: by blocking the production of glutamine, an amino acid that acts as a building block of cells and is used frequently by pancreatic cancers to create more cancer cells. When the Johns Hopkins scientists saw that their nanoparticle-encapsulated version of BPTES shrunk mice tumors by half, Le and her colleagues searched for what major metabolic pathway was driving the growth of the remaining half of the tumor.
To find it, the scientists injected the eight tumor-bearing mice with high levels of labeled glutamine and glucose, another metabolic compound commonly linked to the growth of pancreatic cancer cells. They then traced the compounds' biochemical breakdown through the mice and found that the remaining tumor cells had high amounts of lactate, an end product of the glucose pathway.
With this information, the scientists tested the glucose-blocking anti-diabetes drug metformin, combined with the nanoparticle-encapsulated BPTES, on another eight mice implanted with human pancreatic tumors. The drug combination shrunk tumors by at least 50 percent more than those treated with either drug alone.
Researchers elsewhere have been testing metformin in pancreatic cancer patients with little success, says Le, despite indications that it's a good candidate to treat glucose-dependent tumors. "But it appears the key may be to combine it with other drugs to shut off multiple key pathways in those tumors," she adds.
The scientists have filed a patent for the technology associated with nanoparticle-encapsulated BPTES. The drug's chemical name is bis-2-(5-phenylacetamido-1,2.4-thiadiazol-2yl)ethyl sulfide.
Funding for the research was provided by the National Institutes of Health (CA193895, CA169757, DA032470, MH075673, TR001079).
It's estimated that more than 53,000 people in the U.S. will be diagnosed with pancreatic cancer in 2016. Survival rates are low, with more than 41,000 expected to die of the disease each year.
Additional scientists who contributed to the study include Amira Elgogary, Qingguo Xu, Brad Poore, Jesse Alt, Sarah Zimmermann, Liang Zhao, Jie Fu, Baiwei Chen, Shiyu Xia, Yanfei Liu, Marc Neisser, Christopher Nguyen, Ramon Lee, Joshua Park, Juvenal Reyes, Thomas Hartung, Camillo Rojas, Rana Rais, Takashi Tsukamoto, and Gregg Semenza from Johns Hopkins.

Dr. Laura Wood's New Research Lab
- August 2016
Laura Wood, M..D, Ph.D., one of the talented new physician-scientists in the Sol Goldman Pancreatic Cancer Research Center, just created a new web page describing her pancreatic cancer research lab.
Click the button below, check out Dr. Wood's new research lab, meet her talented team, and learn about her exciting science!
Dr. Wood's research lab website2015 ▼

JHH Researchers receive national and international recognition for their pancreas work
- November 2015
Congratulations to JHH's Dr. Marco Dal Molin! He was awarded the Hirschberg Award for Best Abstract in Pancreatic Cancer at the American Pancreas Association (APA) meeting in San Diego in November 2015. This is one of the key pancreas meetings in the US and was attended by 500 people.
Congratulations to Drs. Anne Marie O’Broin-Lennon and Dr. David Masica who were awarded the Oral Free Paper Prize for the session "Diagnosis and Treatment of Pancreatic Tumours" at the United European Gastroenterology Week (UEGW). This major European GI meeting took place in Barcelona, Spain in October 2015 and was attended by approximately 14,000 people.
A huge congratulations to all and thank you for the work that you do to combat pancreatic cancer!

Johns Hopkins Again Ranked #1 in Pancreatic Cancer Research and Treatment
- April 2015
The website ExpertScape has again ranked Johns Hopkins #1 in pancreatic cancer research and treatment. ExpertScape uses quantitative analysis of publications in the PubMed database to identify the world's foremost experts in pancreatic cancer. In addition to ranking Johns Hopkins #1, three of the top ten ranked experts are from Johns Hopkins (Drs. Hruban, Herman and Wolfgang). Congratulations to the team!

Free Hopkins iPad app
- January 2015
Hopkins releases a free iCarebook iPad app for patients and families. This app is designed to educate and therefore empower patients, family members and friends as they navigate the health care system with a diagnosis of pancreatic cancer. This application is dedicated tot he patients treated at Johns Hopkins and their families.
2014 ▼

Congratulations to Drs. Toby Cornish and Ralph Hruban in the Sol Goldman Pancreatic Cancer Research Center.
- April 2014
Drs. Cornish and Hruban were honored by the Institute for Excellence in Education in the Johns Hopkins University School of Medicine.
They jointly received the 2014 Educational Innovator Award for their work developing novel iPAD and iPhone teaching applications (apps) about pancreatic cancer. One of the apps they developed, The Johns Hopkins Atlas of Pancreatic Pathology, teaches doctors and scientists pancreatic pathology. The second app, The Johns Hopkins iCareBook for Pancreatic Cancer, is designed to help patients and their families facing the diagnosis of pancreatic cancer.

Advances Made in a Blood Test for Pancreatic Cancer
- March 2014
In the February 19, 2014 issue of Science Translational Medicine (Sci Transl Med. 2014 Feb 19;6(224):224ra24), Bettegowda and colleagues in the Sol Goldman Pancreatic Cancer Research center at Johns Hopkins report on an exciting approach to the detection of pancreatic cancer. Bettegowda and colleagues applied cutting edge DNA sequencing to blood samples from a large number of patients with a number of different cancers. They found that many cancers, even some small curable cancers, shed mutant DNA into the blood. This mutant DNA circulating in the blood is called "circulating tumor DNA," or ctDNA for short. The team at Hopkins was able to show that ctDNA is detectable in >80% of patients with advanced pancreatic cancer, and close to half of the patients they studied with early, surgically resectable pancreatic cancers. There is more work to be done, but this study, we believe, represents a significant advance towards the development of a blood test for pancreatic cancer.

Highly Influential Biomedical Researchers
- January 2014
Four members of the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins were identified as "highly influential" biomedical researchers (1996-2011) in a study of published scientific articles. The study tracked the number of times an author's work is cited by other scientists to develop a list of the top 400 living most influential biomedical researchers in the world. Drs. Cameron, Hruban, Kinzler and Vogelstein from the Goldman Center at Johns Hopkins were the only pancreatic cancer researchers on the list. This honor highlights the extraordinary impact of the pancreatic cancer research at Johns Hopkins. Congratulations to Drs. Cameron, Hruban, Kinzler and Vogelstein!
A list of highly influential biomedical researchers, 1996-2011.
Boyack KW, Klavans R, Sorensen AA, Ioannidis JP.
Eur J Clin Invest. 2013 Dec;43(12):1339-65.
PMID:24134636
2013 ▼
Discover expertscape.com
It can be difficult to find a doctor or medical center with the expertise in a particular disease. Dr. John Sotos and colleagues have developed a unique Web-based tool, called ExpertScape, to help patients and their families identify disease-specific experts.
ExpertScape's unique algorithm is based on the idea that true experts are "investigating the leading edge of knowledge and are writing about it." ExpertScape therefore bases its rankings on scientific publications. ExpertScape recently analyzed over 2,900 articles on pancreatic cancer and the Johns Hopkins University came out ranked as the leading expert center on pancreatic cancer. In addition, Dr. Ralph Hruban, the head of the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins was identified as the world's leading expert on pancreatic cancer.
While no system is perfect, it is gratifying to receive this special recognition.

The Ruth Leff Siegel Award goes to Dr. Hruban
Ralph H. Hruban, M.D., head of the Sol Goldman Pancreatic Cancer Research Center received the first The Ruth Leff Siegel Award for Excellence in Pancreatic Cancer Research at a ceremony at Columbia University in New York on September 23, 2013. This $50,000 prize recognizes “the investigator who has made the most significant contribution to the understanding and/or treatment of pancreatic cancer over the past year.”
Congratulations Ralph!




Dr. Anirban Maitra on 60 Minutes
Dr. Anirban Maitra and his student Jack Andraka were featured on a recent episode of 60 Minutes. As a high school student working in Dr. Maitra’s pancreatic cancer research lab, Jack developed a novel approach to detecting molecules in blood. While potentially exciting, it is important to note that the results are unpublished and have not been formally tested in humans. Learn more about this remarkable story.

Pancreatic Cancer Action Network PurpleLight National Vigil For Hope
PurpleLight National Vigil for Hope is a time to honor loved ones fighting pancreatic cancer and those who have lost the fight. Join us in creating awareness of pancreatic cancer and honoring those who have battled this disease, knowing that people all across the United States are doing the same.
During the PurpleLight Ceremony, we will read the names of individuals who have battled the disease, both survivors and loved ones we have lost too soon. Families and friends will be asked to stand and illuminate their purple glow stick when their love one’s name is read.
Date: 10/27/2013
Time: 6:30 pm
Location: Broadway Plaza - Johns Hopkins Outpatient Center
601 North Caroline Street
Baltimore, MD 21287

Johns Hopkins Releases the iCarebook iPad app for Patients and Families
Created by the award-winning APP team from Johns Hopkins (https://www.vesaliustrust.org/2012-netter-award/), the Johns Hopkins iCareBook for Pancreatic Cancer is an educational guide for patients, family members and friends facing a diagnosis of pancreatic cancer. Created by leading experts in the field, this interactive application (app) includes text, illustrations, animations and videos. Emphasis is placed on multi-disciplinary care and the team approach.
The app has three main sections. The MY DISEASE section contains important information on the pancreas and pancreatic cancer, with emphasis on the diagnosis and treatment of the disease. It even contains information on cysts in the pancreas. The MY TEAM section provides information about each of the different specialties that make up the multidisciplinary team that cares for patients with pancreatic cancer. The MY TEAM section can be personalized with the names and pictures of the user's caregivers, and can even integrate with the iOS contacts list. The section can also be used to take notes, such as questions to ask a particular health care specialist. The third section is the MY CARE JOURNAL. This section can integrate with the built-in iOS Calendar and has tools that help to navigate the health care system. In allows the user to track appointments, take notes, record symptoms including pain, and to document medications.
The Johns Hopkins iCareBook for Pancreatic Cancer is designed to educate and therefore empower patients, family members and friends as they navigate the health care system with a diagnosis of pancreatic cancer. The application is dedicated to the patients treated at Johns Hopkins Hospital and their families.

Hopkins Team Recognized for Advancing Pancreatic Cancer Research Through Innovative, Collaborative Science
The Pancreatic Cancer Sequencing Team in the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins received the Team Science Award from the American Association for Cancer Research (AACR) at the AACR's Annual Meeting held in Washington, D.C., on 7th , 2013. This $50,000 award recognizes an outstanding interdisciplinary research team for its innovative and meritorious scientific work that has advanced or will likely advance cancer research, detection, diagnosis, prevention or treatment. The Hopkins team was selected based on its tremendous impact on understanding of the fundamental genetic changes that characterize pancreatic cancer. The interdisciplinary team, led by Ralph H. Hruban, M.D., comprises 20 faculty members from three different institutions: Johns Hopkins University in Baltimore, Md.; Emory University in Atlanta, Ga.; and Memorial Sloan-Kettering Cancer Center in New York, N.Y. Margaret Foti, Ph.D., M.D., chief executive officer of the AACR, said that the team's "research has greatly contributed to our knowledge of pancreatic cancer, which currently has an extremely poor prognosis. They have provided a wonderful example of the innovative scientific discoveries we can expect to find when the efforts of multiple institutions and different biomedical fields collaborate."




PanCAN Meeting
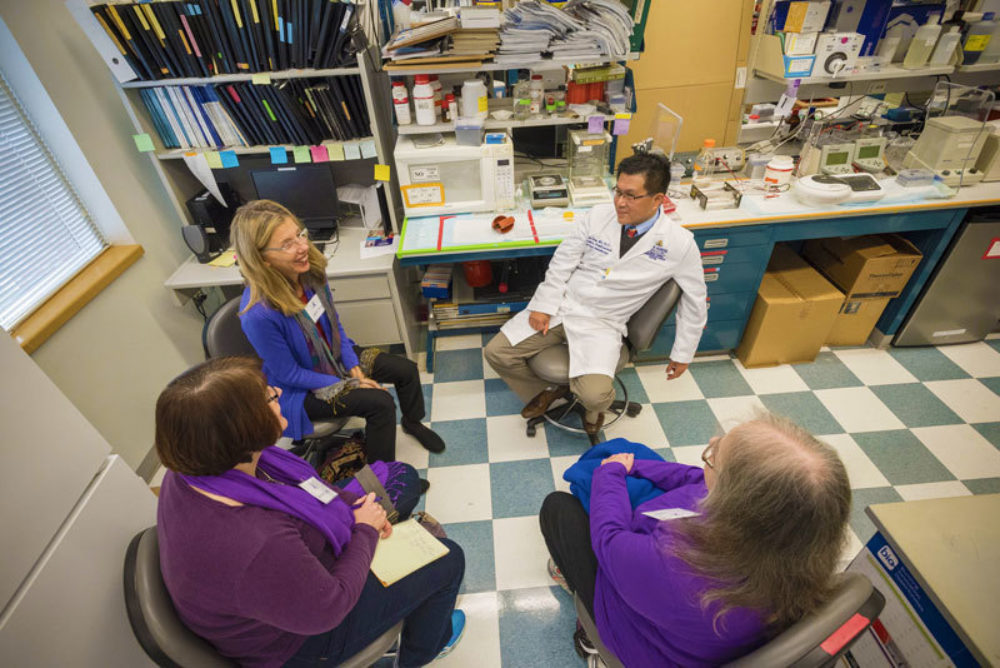
The Baltimore and Washington D.C. affiliates of PanCAN, the national pancreatic cancer advocacy group, visited the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins on Saturday March 24, 2013. Approximately 50 patients, family members and advocates visited. The morning started with welcoming remarks by Teresa Barth, Marsha Balsinger from PanCAN, and an introduction by Dr. Ralph Hruban from the Hopkins team. The visitors then broke up into eleven small groups for small informal meetings with the doctors, scientists and health care providers at Hopkins. Each group spent 10-15 minutes with a member of the Hopkins team, and then moved to the next team member. Dr. Hruban described it as "speed dating!" Most importantly, the PanCAN visitors got a close-up and personal tour of the labs and the opportunity to meet and chat with a number of the world leaders in pancreatic cancer care and research.
The team members from Hopkins who participated in the event included:
- Drs. Lei Zheng & Dan Laheru: Chemotherapy for pancreatic cancer
- Dr. James Eshleman: New approaches to discovering novel therapies
- Elliot Fishman: Recent advances in radiology
- Dr. Michael Goggins: Early detection of pancreatic cancer
- Dr. Joseph Herman: Multidisciplinary treatment of pancreatic cancer
- Mary Hodgin: Gateway to all pancreas services at Johns Hopkins
- Alvin Makohon-Moore & Chelsea Michael: Rapid medical donation program
- Dr. Alison Klein & Diane Echavarria: Familial pancreatic cancer – The NFPTR
- Dr. Matthew Weiss: Surgical treatment of pancreatic cancer
- Ashley Salamone: Pancreatic cyst clinic
- Dr. Na'im Fanaian, Norm Barker & Jon Christopherson: iPad App for Patients (opportunity to be filmed)




2012 ▼
Gift to Sequence DNA of 500 people
The Sol Goldman Pancreatic Cancer Center has received a generous gift to sequence the DNA (genomes) of over 500 people with familial pancreatic cancer. A wonderful example of the impact philanthropy can have, this unique gift will allow scientists at the Goldman Center to define all of the most common inherited DNA changes that predispose to pancreatic cancer. It is hoped that this understanding will, in turn, allow clinicians to predict more accurately a person's risk of developing pancreatic cancer, and that some of the changes will lead to gene-specific treatments for patients who develop pancreatic cancer.
The team at Hopkins has reached out to other centers across North America, and these collaborating centers will contribute samples from research subjects at their institutions. The team at Johns Hopkins is also committed to sharing data generated by this study with other pancreatic cancer scientists, so that the entire research community can benefit from this landmark undertaking. Learn more about familial pancreatic cancer visit and how you can support pancreatic cancer research at Johns Hopkins.


Congratulations Dr. Hanno Matthaei!
Hanno Matthaei MD in the Sol Goldman Pancreatic Cancer Research Center received the "Ferdinand Sauerbruch Research Award" from the Surgical Society of Berlin in a ceremony in Berlin Germany. This 5,000 Euro prize is awarded by the Society for outstanding research by a young surgeon. Dr. Matthaei received this prestigious award for work he conducted in the laboratory of Dr. Anirban Maitra on the molecular pathogenesis and diagnostics of intraductal papillary mucinous neoplasms of the pancreas. Congratulations Hanno!


Congratulations to the pancreatic cancer team at Johns Hopkins!
Thirteen doctors in the Sol Goldman Pancreatic Cancer Research Center were selected by Castle Connolly as Washington-Baltimore "top doctors." They include John Cameron, Marcia Canto, Michael Choti, Elliot Fishman, Deborah Frassica, Michael Goggins, Ralph Hruban, Scott Kern, Daniel Laheru, Steven Leach, Anirban Maitra, Timothy Pawlik, and Ross Donehower.


Fifteen-Year-Old Wins Award
Fifteen-year-old Jack Andraka of Crownsville won the top prize at the Intel International Science and Engineering Fair for designing a new approach to detecting molecules in the blood, Intel announced Friday. Jack has been working in the lab of pancreatic cancer researcher Dr. Anirban Maitra. The top prize is $75,000!


Dr. John L. Cameron performs his 2,000th Whipple
Dr. John L. Cameron, Professor of Surgery at the Johns Hopkins University reached a remarkable milestone. Dr. Cameron performed his 2000th Whipple resection. This milestone not only represents a significant personal accomplishment for Dr. Cameron, but also is a momentous achievement in fight against pancreatic cancer. Dr. Cameron’s work has formed the basis for all of the pancreatic cancer research at Johns Hopkins. In addition, he has trained a generation of surgical residents who themselves have gone out across the country to start their own pancreatic surgery programs. In turn, many of these surgeons have trained additional residents. Dr. Cameron is to be congratulated for reaching this remarkable milestone, and for the lasting legacy he has had providing patients with pancreatic cancer real hope.

Hopkins Team featured in Cell
In the January 20th issue of Cell, Drs. Christine Iacobuzio-Donahue and Joseph Herman from the Sol Goldman Pancreatic Cancer Center, in collaboration with Dr. Franziska Michor at the Dana Farber Cancer Institute, reported on the growth features of pancreatic cancer.
This unique collaboration relied on comprehensive data collected by Dr. Iacobuzio in association with autopsies she performed on patients who died of pancreatic cancer, Dr. Michors expertise in computational modeling of metastasis growth dynamics, and Dr. Herman's expertise in clinical management and treatment of pancreatic cancer patients at Johns Hopkins. First, using data of 101 patients who underwent an autopsy, they characterized the growth dynamics and metastasis of pancreatic cancers and created a mathematical model based on those features. This model was then used to predict a variety of features of pancreatic cancer growth and dissemination in the setting of different treatments, and these findings were then validated in a separate set of 127 patients who had surgery at Johns Hopkins. Together they showed that pancreatic cancers are growing at an accelerated rate when they are diagnosed, that for most patients metastases are already present at diagnosis, and that initiating treatments as soon as possible following diagnosis directly affects patient outcome. The largest implication of their findings is that initiating chemotherapy prior to surgery may offer the greatest chance of survival.
2011 ▼
Johns Hopkins Team Sequences Four Main Cystic Tumors of the Pancreas
Cystic tumors (tumors that forms spaces that contain fluid) of the pancreas can be precancerous. If left in place, some cystic tumors will progress to incurable pancreatic cancer, while others are harmless. These cystic tumors therefore represent on one hand an opportunity to cure pancreatic tumors before they have the opportunity to grow into incurable cancers. On the other hand, since some pancreatic cysts are harmless, these cysts also can be over treated if a harmless cyst is clinically mistaken for a potentially harmful one.
In a real tour de force, the pancreatic cancer research team, led by Dr. Bert Vogelstein, has fundamentally advanced our understanding of the four most common cystic tumors of the pancreas. In findings reported in the December 8th advance on-line edition of the journal Proceedings of the National Academy of Sciences of the United States of America (PNAS), the team announced that they had sequenced all known human genes (>20,000) in 32 cystic tumors of the pancreas: 8 serous cystadenomas (SCA), 8 intraductal papillary mucinous neoplasms (IPMNs), 8 mucinous cystic neoplasms (MCNs), and 8 solid-pseudopapillary neoplasms (SPNs). The team found that each tumor type has its own genetic profile- that is to say that specific genes are targeted in specific tumor types. The VHL gene is targeted in SPNs, the GNAS, RNF43 and KRAS genes in IPMNs, the KRAS, RNF43 and TP53 in MCNs, and the beta-catenin gene in SPNs.
This finding is important for several reasons. First, it provides insight into the fundamental biology of each of these pancreatic tumors. Second, it suggests that the four cysts can be distinguished by their genetic changes. Since the cyst fluid present in these tumors will harbor the same genetic changes as are found in the tumors, this suggests that, in the near future, scientists should be able to diagnose the cyst type just from a small sampling of cyst fluid.

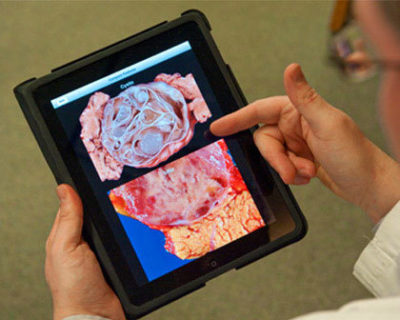
Pancreas Pathology iPad App is Available
We have created a novel tool to teach pancreatic pathology. The Johns Hopkins Atlas of Pancreatic Pathology iPAD application ("app") is a teaching atlas aimed at residents, fellows and practicing pathologists. It contains over 1,400 images and is composed of three modules: an interactive diagnostic algorithm, a searchable image atlas, and an image-based quiz. Viewing multiple examples of the same entity or feature from this large, rich image atlas will strengthen the users diagnostic skills.
The app is available free through the iTunes store. https://itunes.apple.com/us/app/atlas-of-pancreas-pathology/id474845392?mt=8

New Gene Based Tests Distinguish Harmless & Precancerous Cells
Scientists at the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins reported in the July 20 issue of Science Translational Medicine that they have developed a gene-based test that can be used to distinguish harmless from precancerous pancreatic cysts. The test may eventually help patients with harmless cysts avoid needless surgery.
Fluid-filled cysts are actually fairly common in the pancreas. The problem is that it can be hard to distinguish harmless pancreatic cysts from precancerous cysts.
Bert Vogelstein, M.D., and his colleagues discovered that almost all of the precancerous cysts of the pancreas have mutations in the GNAS and/or the GNAS gene. The researchers then tested a total of 132 precancerous pancreatic cysts for mutations in KRAS and GNAS. Nearly all (127) had mutations in GNAS, KRAS or both. Next, the investigators tested harmless cysts, and the harmless cysts did not have GNAS or KRAS mutations Larger numbers of patients must be studied before the gene-based test can be widely offered. https://pathology.jhu.edu/pancreas/cyst-clinic
Learn more about Pancreatic Cysts
Scientists at Hopkins Make a Major Advance in the Understanding of Pancreatic Neuroendocrine Tumors
Christopher Heaphy, Roeland De Wilde and Yuchen Jiao in the laboratory of Alan Meeker in the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins reported today (June 30th, 2011) in the on-line edition of the journal Science that they have discovered that pancreatic neuroendocrine tumors have unique chromosome changes called "Alternative Lengthening of Telomeres." Alternative Lengthening of Telomeres affects the ends of chromosomes, the part of the chromosomes called telomeres. The team then showed that Alternative Lengthening of Telomeres in pancreatic neuroendocrine tumors (also known as "islet cell tumors") is likely caused by mutations (DNA changes) in the DAXX and ATRX genes. This finding is important because it uncovers an entirely new cancer pathway. Now that this new pathway has been discovered, the team hopes that this pathway can be exploited diagnostically and therapeutically.


New Book from Johns Hopkins University Press on Pancreatic Cancer
Dr. Dung T. Le, a medical oncologist at Johns Hopkins, and her patient Michael J. Lippe have teamed together and written a remarkable book on pancreatic cancer. Michael was diagnosed with stage IV pancreatic cancer in 2007, and the book, "Pancreatic Cancer: A Patient and His Doctor Balance Hope and Truth," (Johns Hopkins University Press) chronicles Michael's journal through his diagnosis and treatment. Michael and Dr. Le alternate chapters, providing two perspectives on the disease and on their partnership fighting Michael's illness. Michael and Dr. Le hope that "their honest yet hopeful persoective will help all people with cancer and those who care for them."
View the book here on Amazon.

Exctiting Trial with FOLFIRINOX
The May 12, 2011 New England Journal of Medicine (N Engl J Med 364;19) contains a report by Thierry Conroy, M.D. and colleagues from the Groupe Tumeurs Digestives of Unicancer and the PRODIGE Intergroup in France describing the results of an exciting clinical trial of the drug combination known as "FOLFIRINOX." In this large study 342 patients were randomized to receive FOLFIRINOX or gemcitabine (Gemzar). Six months of chemotherapy were recommended in both groups in patients who had a response, and the primary end point of the study was overall survival. The patients who received FOLFIRINOX did better than the patients who received gemcitibine. The median overall survival was 11.1 months in the FOLFIRINOX group as compared with 6.8 months in the gemcitabine group, and the median progression-free survival was 6.4 months in the FOLFIRINOX group and 3.3 months in the gemcitabine group. 31% of the patients who received FOLFIRINOX had an objective response versus 9.4% in the gemcitabine group. The treatment is not without its draw backs as the FLORFIRINOX group did have some side effects of therapy, and some of these were serious.
This important studies demonstrates some of the exciting progress that is being made in the treatment of pancreatic cancer.

Islet cell tumors and Endocrine Tumors Sequenced
Investigators in the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins have sequenced all known human genes in a series of ten pancreatic neuroendocrine tumors of the pancreas (also known as islet cell tumors and endocrine tumors). This article appears in the January 20th advance on-line issue of Science magazine called Science Express. This paper is exciting for three reasons:
- The investigators found that one-sixth of pancreatic neuroendocrine tumors of the pancreas have mutations in genes coding for proteins in the mTOR pathway. There are drugs currently available (rapalogues such everolimus) that specifically target the mTOR pathway. The findings suggest that doctors should be able select only those patients who will benefit from an mTOR inhibitor (patients whose tumors have a mutation in the mTOR pathway), while sparing patients who would not benefit because their tumors do not have mutations in this pathway.
- The investigators discovered a new cancer pathway. They discovered mutations in two genes (ATRX and DAXX) that have not been reported before in any tumor. These are exciting as they provide insight into the fundamental biology of neuroendocrine tumors of the pancreas, and, as scientists learn more about the function of these genes, we hope completely novel therapies.
- The scientists found that tumors with both ATRX/DAXX mutations and mutations in another gene (MEN-1) have a significantly better prognosis than do tumors that lack these mutations.
We believe that this sequencing project represents a significant advance in the war against pancreatic neuroendocrine tumors. The results have both immediate and long-term impact.

New Approach to the Early Detection of Cancer
A team of scientists at Johns Hopkins, led by Drs. Bert Vogelstein and Akhilesh Pandey, have developed a new approach to the early detection of cancer, including pancreatic cancer. This advance was published on January 19th in the on-line early edition of the journal Proceedings of the National Academy of Sciences (PNAS, https://www.pnas.org/content/early/recent). Since DNA codes for RNA which in turns codes for proteins, Drs. Pandey and Vogelstein reasoned that they should be able to detect mutations in DNA by carefully studying proteins. Indeed they were. Briefly, using cutting-edge technologies to examine proteins the team was able to show that they can detect very small quantities of mutant proteins. This advance is important because cancer, including pancreatic cancer, is fundamentally caused by DNA mutations (mistakes in the DNA). These mutations (mistakes) can now be detected at the protein level.
2010 ▼
Frailty Score
Surgeons at Johns Hopkins in collaboration with the geriatrician Linda Fried at Columbia University have developed a new "Frailty Score" which predicts how older patients will weather the stresses of surgery. This advance was published in The Journal of the American College of Surgeons and reported on in a recent issue of the New York Times. This new score helps surgeons assess their patients' risks of surgery. Rather than simply relying on a patient's age, surgeons can now use this new tool to balance the risks and benefits of surgery in older patients.

Hopkins Scientist Use Knowledge of Genetic Changes to Guide Therapy
In an advance on-line issue of the journal Molecular Cancer Therapeutics Dr. Villarroel and colleagues from Johns Hopkins report the case of a patient whose successful treatment was guided by the specific genetic changes (mutations) in their pancreatic cancer. The patient described had gemcitabine-resistant metastatic pancreatic cancer. This patient was treated with the drug Mitomycin C based on laboratory studies of his cancer. Dr. Villarroel and colleagues report that the patient had a dramatic long lasting (36+ months) tumor response. Furthermore, Dr. Villarroel and colleagues go on to show that a specific rare genetic change in the patient’s pancreatic cancer, a PALB2 gene mutation, was responsible for their tumor’s responsiveness to Mitomycin C. This work is exciting for two reasons. First, it suggests that inactivation of the PALB2 gene is a determinant of response to Mitomycin C and other chemotherapy treatments that work by initiating DNA damage in tumors. Second, it suggests that sequencing the genes in a cancer can identify new targets for "personalized cancer care."
Disclosure of Potential Conflicts of Interest:
Siân Jones, Ralph H. Hruban, James R. Eshleman and Alison Klein are co-authors on this paper and they are also co-inventors on PLAB2- related intellectual property managed by Johns Hopkins University and have the potential to receive royalty payments for the PALB2 invention.


Genetic Study Reveals Large Window of Opportunity for the Early Detection of Pancreatic Cancer
Pancreatic cancer develops and spreads much more slowly than scientists have thought, according to new research from the laboratory of Dr. Christine Iacobuzio-Donahue M.D. Ph.D., Associate Professor of Pathology, Oncology and Surgery at Hopkins' Sol Goldman Pancreatic Cancer Research Center. Using whole genome sequencing data generated from the cancer tissues of seven patients who underwent a rapid autopsy, Dr. Iacobuzio and colleagues found that it takes at least a decade after the first cancer-causing mutation occurs in a normal cell in the pancreas until the development of a full-fledged cancer cell. After the first cancer cell appears, it takes an average of nearly seven years for that cell to turn into the billions that make up a cancerous tumor diagnosed by imaging methods, after which at least one of the cells within the tumor has the potential and ability to spread to other organs. Patients die an average of two and a half years after this metastasis. The study, published in the October 28th issue of the journal Nature, contradict the idea that pancreatic cancers metastasize very early in their development, and indicates that there is a potentially broad window for diagnosis and prevention of the disease.
"For the first time, we have a quantifiable estimate of the development of pancreatic cancer, and when it would be best to intervene," according to Dr. Iacobuzio, "so there is potentially a very broad window for screening." Right now, however, she adds, "pretty much everybody is diagnosed after that window has closed."
More on the paper can be found at Nature.com.
2009 ▼
PANCAN's Purple Stride Maryland
- October, 2009
On Sunday October 11,2009, Team Hopkins comprised of Diane Echavarria, Coordinator of the National Familial Pancreas Tumor Registry, Brian Kirby and Alexis Kirby, Research Specialist in Dr. James Eshleman's lab, ran the 5K fun run in Oregon Ridge Park in support of PANCAN's Purple Stride Maryland-2009. The team helped raise money and awareness for pancreatic cancer by collecting donations in support of their run in addition to making winning bids on items in Purple Stride's Silent Auction.






PanCan Visit
- September, 2009
Close to 60 members of the Baltimore and Washington D.C. branches of Pancreatic Cancer Action Network (PanCAN) visited the pancreatic cancer research labs at Johns Hopkins on Saturday September 12th. The event was organized by Mary Zapor and Marsha Garil (Co-Coordinators of PanCAN’s National Capital Area Affiliate). The day started bright and early with a meet and greet breakfast which was followed by welcoming remarks by Mary Zapor, Marsha Garil, and an introduction by Dr. Ralph Hruban. From there the PanCAN visitors split into ten small groups for multiple informal sessions with the Hopkins' doctors and scientists. One of the visitors from PanCAn described the day saying' "They sat down with us, looked us in the eye and explained what they were doing on OUR level." At the end of the day Mary Zapor summarized the experience with "these docs rock!"











The Team at Johns Hopkins Discovers Genetic Marker of Prognosis
- September, 2009
Scientists at Johns Hopkins combed through all 21,000 known human genes to identify the genetic changes (mutations) in pancreatic cancer that are associated with prognosis. Using data from the pancreatic cancer genome initiative conducted at Johns Hopkins, the investigators were able to show that cancers which harbor mutations in the DPC4 (also known as SMAD4) gene have a worse prognosis after surgical resection than do cancers with normal (intact or "wild-type") DPC4. These results suggest that genetic changes may be useful markers for patient prognosis. The results of this study appeared in the July 15th, 2009 issue of Clinical Cancer Research, PMID: 19584151.

Scientist at Johns Hopkins Team with Scientists in Cambridge to Identify Cause of Drug Resistance in Pancreatic Cancer
- May, 2009
In the May 14th on-line issue of Science magazine scientists from the Cambridge Research Institute in the United Kingdom, together with scientists from the Sol Goldman Pancreatic Cancer Research Center, report that poor blood flow may explain some of the insensitivity of pancreatic cancer to treatment. Kenneth Olive and colleagues found that there are very few blood vessels in most human pancreatic cancers, and as a result, the cancers are poorly perfused and little chemotherapy enters the tumors. The team then tested the drug IPI-926 in a mouse model of pancreatic cancer. This drug depletes some of the stroma (scar tissue) within pancreatic cancers by inhibiting the Hedgehog cellular signaling pathway. The scientists found that combined treatment of pancreatic cancers in mice with the drug IPI-926 and with gemcitabine produced an increase in the blood flow to tumors, an increase in drug deliver to the tumors, and stabilization of disease. This study is exciting because it suggests that inefficient drug delivery may be an important contributor to chemoresistance in pancreatic cancer. Finding the cause of a problem is an important step in overcoming the hurdle.

Work at Johns Hopkins for Early Detection of Cancer
- May, 2009

Victor Velculescu, M.D., Ph.D.
Romanian-born Victor Velculescu has won the 2009 Outstanding Achievement Award in Cancer Research from the American Association of Cancer Research (AACR). The award recognizes young investigators for their contributions to the field. He will receive his prize and give a lecture at the AACR annual meeting on April 22 in Denver.
Velculescu (pronounced Vel-ku-les-ku) pinpointed the PIK3CA gene as one of the most frequently mutated genes ever identified in human cancer. He also developed a method to rapidly identify disease-related genes and measure gene expression called SAGE (Serial Analysis of Gene Expression), and, with his colleagues, developed the first draft genome sequence of the four human cancer types: breast, colorectal, pancreatic and an aggressive form of brain cancer called glioblastoma multiforme.

Bert Vogelstein, M.D.
Bert Vogelstein, M.D. whose published studies of cancer genetics are the most highly cited works in the field, will receive this year’s American Society of Clinical Oncology "Science of Oncology" Award at the group’s annual meeting in Orland, Fla., on June 1, 2009.
Vogelstein was selected for his role in discovering the specific genes and mutations responsible for colorectal cancer and for establishing a genetic model that explains how most solid tumors form and progress. In addition to discovering these genes, Vogelstein and his team have developed blood tests which are now used to identify patients with inherited mutations in the genes linked to colorectal cancer. They are currently working on the development of non-invasive methods to detect individuals who have early, curable colorectal tumors and to design therapies based on the new understanding of the molecular basis of cancer.
In the past year, Vogelstein and colleagues mapped the complete genetic blueprints for pancreatic and brain cancer, two of the most deadly tumor types. And they completed the first genome maps for breast cancer and colorectal cancer in 2007.
Media contact: Amy Mone, 410-955-1287

Scientists at Johns Hopkins Receive a Large Grant to Follow-up on the Goldman Pancreatic Cancer Genome Project
- May, 2009
Investigators in the Sol Goldman Pancreatic Cancer Research Center, the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, and the McKusick-Nathans Institute of Genetic Medicine have been awarded an $8 million dollar P01 Program Project Grant (PPG) from the National Cancer Institute. This program, entitled "Functional Annotation of the Pancreatic Cancer Genome", will seek to functionally characterize novel oncogenes and tumor suppressor genes identified through next generation sequencing of human pancreatic cancer genomic DNA, using cell-, zebrafish- and mouse-based model systems. The program is directed by Dr. Steven Leach, and includes individual projects directed by Dr. Leach, Dr. Scott Kern, Dr. Anirban Maitra and Dr. Joshua Mendell. The grant also supports both a Zebrafish Core, directed by Dr. Michael Parsons, and a Phenotyping Core, directed by Dr. David Huso. The effort builds on the Pancreatic Cancer Genome Sequence project spearheaded by Dr. Ralph Hruban, Dr. Bert Vogelstein and Dr. Victor Velculescu, and is ultimately made possible by the high volumes of pancreatic cancer patients cared for by specialists in the Departments of Surgery, Oncology, and Radiation Oncology.

DNA Changes Caused By Smoking
- April, 2009
Scientists at the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins report in the April 15th issue of Cancer Research (Cancer Research volume 69, pages 3681-3688, 2009) that up to 25% of the genetic mutations found in pancreatic cancers from cigarette smokers are caused by cigarette smoking.
Smoking cigarettes is known to double the risk of pancreatic cancer, and cigarette smoking is believed to account for 20% to 25% of pancreatic cancers. The recent sequencing of the pancreatic cancer genome provided the team at Johns Hopkins a unique opportunity to discover the DNA changes (mutations) caused by cigarette smoking. The scientists compared the DNA mutations in pancreatic cancers obtained from individuals who smoked cigarettes to the DNA mutations in pancreatic cancers obtained from individuals who never smoked cigarettes. They found more DNA mutations in the pancreatic cancers obtained from smokers (average 53.1 mutations per tumor) than in the pancreatic cancers obtained from never smokers (average 38.5 mutations per tumor). The types and patterns of these mutations provide insight into the mechanisms by which cigarette smoking causes pancreatic cancer.

Team at Johns Hopkins to Partner with team from Australia to Sequence 500 Pancreatic Cancers
- April, 2009
The Australian Governement has committed $27.5 million (Australian dollars) to sequence completely 500 pancreatic cancers as part of the International Cancer Genome Consortium. The work will be led by Sean Grimmond of the University of Queensland, one of Australia’s leading genome sequencing experts, and by Professor Andrew Biankin, who heads a pancreatic cancer research group at Sydney’s Garvan Institute. The team from the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins, including Drs. Anirban Maitra and Ralph Hruban, will collaborate with the Australians, providing critical biosamples needed for the project.
This project builds on the recent sequencing of the pancreatic cancer genome by the Hopkins team, and it is a wonderful demonstration of international collaboration in the war against pancreatic cancer.
To learn more, download pdf

Dr. Iacobuzio-Donahue wins Ramzi Cotran Prize for Her Pancreatic Cancer Research
- March, 2009
Congratulations Dr. Christine Iacobuzio-Donahue, winner of the 2009 Ramzi Cotran Young Investigator Award. This award was presented to Dr. Iacobuzio-Donahue at the 98th Meeting of the United States and Canadian Academy of Pathology in Boston, MA. The award recognizes a body of work by a scientist under the age of 45 which has contributed significantly to the diagnosis and understanding of human disease. Dr. Iacobuzio-Donahue was awarded this prestigious award specifically for her pancreatic cancer research, including her discoveries into why some pancreatic cancers metastasize (spread).

Hopkins Scientists Discover a New Familial Pancreatic Cancer Gene
- March, 2009
Scientists at the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins are the first to discover a disease-causing gene by sequencing all 21,000 known human genes from a single individual, a patient with familial pancreatic cancer. The team, led by Dr. Alison Klein, Ph.D., compared the changes observed in the patient’s DNA to those found in healthy individuals and identified a single mistake in a gene, called PALB2, that was responsible for this patient's familial pancreatic cancer. Their findings, which have implications for all genetic diseases, not just pancreatic cancer, are reported in the March 5 edition of Science Express.
Both the patient sequenced and his sister developed pancreatic cancer, suggesting an inherited predisposition. It would have been impossible to identify the gene responsible for such a predisposition with conventional techniques, which generally require very large families. So the investigators sequenced all the genes (close to 21,000!), hoping that they'd find the responsible alteration. "Gene sequencing has always had the potential to help us learn if a person is susceptible to certain diseases," says Alison Klein, Ph.D., director of the National Familial Pancreas Cancer Tumor Registry at Johns Hopkins. "By finding the genetic error responsible for this patient’s familial pancreatic cancer, our team has provided an excellent example of the power of this approach."
This study was based on the Goldman Center's recently completed pancreatic cancer genome sequencing project. Specifically, the team reviewed all of the DNA sequences from the individual whose brother also had pancreatic cancer. With the help of high-powered computer software, the team scanned all known protein-coding genes -- approximately 21,000 of them – to find variations in this individual. "Most of the variations were normal ones coding for such things as eye or hair color, but the search was designed to track down particular mutations that caused certain proteins to be shortened, a process that commonly occurs in cancers," says James Eshleman, M.D., Ph.D., Associate Professor of Pathology and Oncology. This search led to a variant in one gene, called PALB2, that resulted from a substitution of a DNA base cytosine with a different one, thymidine.
The research team then looked for PALB2 gene alterations in 96 other individuals with pancreatic cancer, each of whom at least one additional relative with pancreatic cancer. They found that three of these 96 individuals had errors in the PALB2 gene that resulted in similar shortening of the protein product. Interestingly, the PALB2 protein binds to the BRCA2 protein, the product of another breast and pancreatic cancer susceptibility gene. Learn more about PALB2, BRCA2 and other causes of familial pancreatic cancer.
The investigators believe that their approach could be used to identify inherited alterations that predispose to many other types of cancers as well as other diseases with genetic components. Sequencing of all the genes in a cancer and in person's genome has the potential to discover the inherited genes that predispose to the cancer, as well as the acquired genetic changes that allow that cancer to blossom. If funding can be found, the team would like to try this approach on other cancer types. They would like to sequence a series of well-characterized islet cell tumors of the pancreas.
In addition to Klein, Eshleman, Goggins and Vogelstein, investigators who conducted the research included Siân Jones, Ralph Hruban, Mihoko Kamiyama, Michael Borges, Xiaosong Zhang, D. Williams Parsons, Jimmy Cheng-Ho Lin, Emily Palmisano, Keiran Brune, Elizabeth Jaffee, Christine Iacobuzio-Donahue, Anirban Maitra, Giovanni Parmigiani, Scott Kern, Victor Velculescu, and Kenneth Kinzler of the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins.

Dr. Makary on ABC News
- February, 2009
Dr. Makary answers questions about supreme court justice Ruth Bader Ginsburg's surgery on ABC News.
Supreme Court Justice Ginsberg has pancreas surgery


Dr. Akhilesh Pandey featured as one of the breakthrough technology leaders of Baltimore
- January, 2009
Congratulations to Johns Hopkins pancreatic cancer research team member Dr. Akhilesh Pandey for being featured as one of the breakthrough technology leaders of Baltimore - it is part of the cover story for January's Urbanite magazine.
Dr. Pandey is a leader in the field of proteomics and his team is dedicated to developing am early detection test for pancreatic cancer.
Congratulations Akhilesh!
2008 ▼
National Familial Pancreas Tumor Registry Website
- December, 2008
Although the NFPTR registry is not new (we’ve been in existence since Dr. Hruban founded the registry in 1994), the NTPTR website is now totally redesigned! Check it out for information on the purpose of our research, insights gained, and new research studies you may be eligible to participate in!
If you haven’t heard about us before, the NFPTR is a research study aimed at identifying the causes of pancreatic cancer. We hope that our research will enable the early detection of pancreas cancer and lead to improved treatment of this disease, ultimately saving lives. Over 3000 families are currently part of our registry. While many families have enrolled, each family that joins teaches us more about this disease, such that the addition of more families to our study is critical to continue our progress. You can join the NFPTR if you or a family member has been diagnosed with a pancreatic tumor. Participation involves the completion of a questionnaire about your health history and that of your family members, and potentially the optional donation of a research DNA sample.
Visit our new NTPTR website to:
- See if you’re eligible to participate in the study
- Learn more about familial pancreas cancer risk
- Ask questions
- Connect with other patients and caregivers
- Read our annual newsletter
- Meet the NFPTR team
- Directly request a questionnaire to join the study
You can also contact the study coordinator directly at 410-955-3502 or [email protected].

Combining Pancreatic Cancer Surgery with Vaccine
- October, 2008
Dr. Martin Makary has written a new blog post on the pancreas, called Where is the Pancreas?, which discusses some important basic information about the pancreas. Dr, Makary works with the Johns Hopkins Pancreas and Advanced Laparoscopic Surgery departments.
Read more about the pancreas and its location.

Combining Pancreatic Cancer Surgery with Vaccine
- September, 2008
The Department of Surgical and Medical Oncology announces the next clinical trial that will test the safety and immune activity of an allogeneic GM-CSF-secreting pancreatic cancer vaccine for patients who are eligible for pancreatic cancer surgery. This trial will incorporate the pancreatic cancer vaccine with surgical resection as part of combinatorial therapy for pancreatic cancer. This pancreatic cancer vaccine was developed by the Johns Hopkins pancreatic cancer vaccine team in the Department of Medical Oncology at The Johns Hopkins Hospital; and more than 200 patients with different stages of pancreatic cancer have received this vaccine through clinical trials. Prior studies have demonstrated that this vaccine is safe and and induces immune responses to pancreatic cancer. In this new study the vaccine treatment will be given two weeks prior to surgery and then multiple times after surgery along with chemotherapy and radiation after surgery. Patients with resectable pancreatic cancer who qualify for this clinical trial will be able to receive their first vaccine as early as two weeks prior to the surgery.

Genetic Blueprint for Pancreatic Cancer
- September, 2008
In an exciting advance, the complete genetic blueprint for pancreatic cancer, one of the most lethal of all of the cancers, was decoded by a team at the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins. The study, led by Drs. Vogelstein, Kinzler and Velculescu, is reported in the Sept. 5, 2008, issue of Science Express.
The team sequenced more than 20,000 genes in a series of 24 well-characterized pancreatic cancers and discovered over 1,500 DNA mutations in these cancers. An average of 63 mutations was found in each cancer, supporting the growing body of evidence that cancer is fundamentally a disease caused by alterations in the DNA. The complex picture presented by these mutations was simplified by the finding that many of them acted in concert through a set of well-defined signaling pathways and processes. The scientists identified 12 core signaling pathways and processes that were each altered in more than two-thirds of the cancers. These 12 core pathways provide the basis for novel diagnostic and therapeutic approaches in pancreatic cancer.
As a part of the study the team also discovered over 500 genes that were made at abnormally high levels in the 24 cancers. Fifty-four of these over expressed genes were predicted to be secreted or made on the surface of the cancer cells, suggesting that these genes may be useful therapeutic targets or may form the basis for new tests for the early detection of pancreatic cancer.
The landmark study characterizes the fundamental genetic components of pancreatic cancer and will guide research on this disease for the next decade. The improved understanding realized from these studies, and their follow-up work will hopefully lead to dramatic improvements in the prevention, detection, or treatment of pancreatic cancer.
The project is also a great example of the power of private giving. The major funding The Pancreatic Cancer Genome Project came from the Sol and Lillian Goldman Charitable Trusts. Significant funding also came from The Lustgarten Foundation for Pancreatic Cancer Research. Additional funding came from the Virginia and D. K. Ludwig Fund, Susan G. Komen Foundation, Michael Rolfe Pancreatic Cancer Foundation, Joseph C. Monastra Foundation, the family and friends of George Rubis, Viragh Family Foundation, Broad Foundation, Emerald Foundation, and National Institutes of Health.

New FTC campaign launched
- August 2008
The Federal Trade Commission launched a campaign today to increase consumer awareness about cancer treatment hoaxes. The campaign includes a Web site, banner ads, a two-minute video, educational materials, and a consumer alert.
The website is https://ftc.gov/curious.
Also note that the Johns Hopkins Cancer Center's Web site: The Sidney Kimmel Comprehensive Cancer Center has information under its Latest News column about two email hoaxes related to Johns Hopkins.


Pancreatic Cancer Blog
- August, 2008
We have created a new blog on pancreatic cancer!
We created this blog to facilitate communication with patients, their families and friends as they face health issues related to the pancreas. Our plan is to have our experts regularly post blogs on "hot issues." We hope that you find these blogs interesting and educational, and we encourage you to contribute your thoughts, experiences and expertise to our on-line blog. The blog is something new for us, so we appreciate your patience as we iron out any issues that may appear.
Enjoy!

This e-mail is NOT from Johns Hopkins
- August 2008
A hoax e-mail on cancer has been circulating on the internet. Because this e-mail uses the Johns Hopkins name, we thought we would post a What's New to inform the users of our web page. The e-mail in question is pasted at the end of this Whats New. To learn more visit: www.jhsph.edu/dioxins and The Sidney Kimmel Cancer Center News.
The hoax email in question:
AFTER YEARS OF TELLING PEOPLE CHEMOTHERAPY IS THE ONLY WAY TO TRY (TRY THE KEYWORD) AND ELIMINATE CANCER, JOHN HOPKINS IS FINALLY STARTING TO TELL YOU THERE IS AN ALTERNATIVE WAY.
Cancer Update from John Hopkins
- Every person has cancer cells in the body. These cancer cells do not show up in the standard tests until they have multiplied to a few billion. When doctors tell cancer patients that there are no more cancer cells in their bodies after treatment, it just means the tests are unable to detect the cancer cells because they have not reached the detectable size.
- Cancer cells occur between 6 to more than 10 times in a person's life time
- When the person\'s immune system is strong the cancer cells will be destroyed and prevented from multiplying and forming tumors.
- When a person has cancer it indicates the person has multiple nutritional deficiencies. These could be due to genetic, environmental, food and lifestyle factors.
- To overcome the multiple nutritional deficiencies, changing diet and including supplements will strengthen the immune system.
- Chemotherapy involves poisoning the rapidly-growing cancer cells and also destroys rapidly-growing healthy cells in the bone marrow, gastro-intestinaltract etc, and can cause organ damage, like liver, kidneys, heart, lungs etc.
- Radiation while destroying cancer cells also burns, scars and damages healthy cells, tissues and organs.
- Initial treatment with chemotherapy and radiation will often reduce tumor size. However prolonged use of chemotherapy and radiation do not result in more tumor destruction.
- When the body has too much toxic burden from chemotherapy and radiation the immune system is either compromised or destroyed, hence the person can succumb to various kinds of infections and complications.
- Chemotherapy and radiation can cause cancer cells to mutate and become resistant and difficult to destroy. Surgery can also cause cancer cells to spread to other sites.
- An effective way to battle cancer is to starve the cancer cells by not feeding it with the foods it needs to multiply.
- Meat protein is difficult to digest and requires a lot of digestive enzymes. Undigested meat remaining in the intestines become putrified and leads to more toxic buildup.
- Cancer cell walls have a tough protein covering. By refraining from or eating less meat it frees more enzymes to attack the protein walls of cancer cells and allows the body\'s killer cells to destroy the cancer cells.
- Some supplements build up the immune system (IP6, Flor-ssence, Essiac, anti-oxidants, vitamins, minerals, EFAs etc.) to enable the body's own killer cells to destroy cancer cells. Other supplements like vitamin E are known to cause apoptosis, or programmed cell death, the body's normal method of disposing of damaged, unwanted, or unneeded cells.
- Cancer is a disease of the mind, body, and spirit. A proactive and positivespirit will help the cancer warrior be a survivor. Anger, unforgiveness and bitterness put the body into a stressful and acidic environment. Learn to havea loving and forgiving spirit. Learn to relax and enjoy life.
- Cancer cells cannot thrive inan oxygenated environment. Exercising daily, and deep breathing help to get more oxygen down to the cellular level. Oxygen therapy is another means employed to destroy cancer cells.
Cancer Cells Feed On:
- Sugar is a cancer-feeder. By cutting off sugar it cuts off one important food supply to the cancer cells. Sugar substitutes like NutraSweet, Equal, Spoonful, etc are made with Aspartame and it is harmful. A better natural substitute would be Manuka honey or molasses but only in very small amounts. Table salt has a chemical added to make it white in color. Better alternative is Bragg's aminos or sea salt.
- Milk causes the body to produce mucus, especially in the gastro-intestinaltract. Cancer feeds on mucus. By cutting off milk and substituting with unsweetened soya milk cancer cells are being starved.
- Cancer cells thrive in an acid environment. A meat-based diet is acidic and it is best to eat fish, and a little chicken rather than beef or pork. Meat also contains livestock antibiotics, growth hormones and parasites, which are all harmful, e specially to people with cancer.
- A diet made of 80% fresh vegetables and juice, whole grains, seeds, nuts and a little fruits help put the body into an alkaline environment. About 20% can be from cooked food including beans. Fresh vegetable juices provide live enzymes that are easily absorbed and reach down to cellular levels within 15 minutes to nourish and enhance growth of healthy cells. To obtain live enzymes for building healthy cells try and drink fresh vegetable juice (most vegetables including bean sprouts)and eat some raw vegetables 2 or 3 times a day. Enzymes are destroyed at temperatures of 104 degrees F (40 degrees C).
- Avoid coffee, tea, and chocolate, which have high caffeine. Green tea is a better alternative and has cancer-fighting properties. Water-best to drink purified water, or filtered, to avoid known toxins and heavy metals in tap water. Distilled water is acidic, avoid it.
(PLEASE FORWARD IT TO PEOPLE YOU CARE ABOUT)
CANCER UPDATE FROM JOHN HOPKINS HOSPITAL , U S - PLEASE READ
- No plastic containers in micro.
- No water bottles in freezer.
- No plastic wrap in microwave.
Johns Hopkins has recently sent this out in its newsletters. This information is being circulated at Walter Reed Army Medical Center as well.
Dioxin chemicals causes cancer, especially breast cancer.
Dioxins are highly poisonous to the cells of our bodies.
Don't freeze your plastic bottles with water in them as this releases dioxins from the plastic.
Recently, Dr. Edward Fujimoto, Wellness Program Manager at Castle Hospital, was on a TV program to explain this health hazard. He talked about dioxins and how bad they are for us.. He said that we should not be heating our food in the microwave using plastic containers.
This especially applies to foods that contain fat. He said that the combination of fat, high heat, and plastics releases dioxin into the food and ultimately into the cells of the body. Instead, he recommends using glass, such as CorningWare, Pyrex or ceramic containers for heating food. You get the same results, only without the dioxin. So such things as TV dinners, instant and soups, etc., should be removed from the container and heated in something else.
Paper isn't bad but you don't know what is in the paper. It's just safer to use tempered glass, Corning Ware, etc. He reminded us that a while ago some of the fast food restaurants moved away from the foam containers to paper. The dioxin problem is one of the reasons.
Also, he pointed out that plastic wrap, such as Saran, is just as dangerous when placed over foods to be cooked in the microwave. As the food is nuked, the high heat causes poisonous toxins to actually melt out of the plastic wrap and drip into the food. Cover food with a paper towel instead.
This is an article that should be sent to anyone important in your life.

Dr. Randy Pausch dies
- July 2008
It is with great sadness that we note the passing of Dr. Randy Pausch, Carnegie Mellon's Last Lecture professor, at age 47. He died at his home in southern Virginia.

Farewell to Randy Pausch
On behalf of the many members of the Sol Goldman Research Team, we wish to extend our condolences to the Family and Friends of Randy Pausch. Randy understood the seriousness of advanced pancreatic cancer as well as the challenges and the realities that he and many others with this cancer must face. Through his initial desire to share his wisdom and his experiences to his wife and children, he managed to speak for many of these same patients and share his message of living a fulfilled life to the world. Randy clearly had an amazing gift of clarity and purpose and generously gave of his time to speak to Congress and others about the critical nature of basic science and clinical research as the mechanism to better understand this cancer and ultimately find better treatments for pancreatic cancer.
We remain committed in our mission to identify how pancreas cancer develops and grows with the goal in the end that we will be able to develop better treatments for this cancer so others will not have to go through what Randy and so many others had/have to do. We think this will be how our group can best honor the memory of Randy.

Hopkins Scientists Create a Fish Model of Pancreatic Cancer
- June 2008
One of the major road blocks to the study of pancreatic cancer has been the absence of a good animal model of this disease. Scientists at Johns Hopkins previously collaborated with Dr. Tuveson in the creation of the first mouse model of pancreatic cancer. This week, Dr. Steven Leach and colleagues at Johns Hopkins announced that they have created a fish model of pancreatic cancer. Dr. Leach and colleagues generated the fish by inserting mutant KRAS genes into the fish pancreas. KRAS is the gene most commonly mutated in human pancreatic cancer. The cancers that developed in the fish showed several features in common with the human disease. The authors conclude that their results provide a unique view of the tumor-initiating effects of the KRAS gene in a living vertebrate organism, and suggest that zebrafish models of pancreatic cancer may prove useful in advancing our understanding of the human disease.

Johns Hopkins Pancreas Multidisciplinary Cancer Clinic
- June 11, 2008
In the Annals of Surgical Oncology Dr. Timothy Pawlik and colleagues from Johns Hopkins report the findings from the first year of the Johns Hopkins Multidisciplinary Pancreatic Cancer Clinic. They compared the clinical care recommendations generated by the multidisciplinary clinic with the recommendations the patients received prior to coming to the clinic. Remarkably, 18.7% of the patients had a change in the status of their clinical stage. Review of the histological slides by dedicated pancreatic pathologists resulted in changes in the interpretation in another 3.4% of the patients. Overall, 23.6% of the patients had a change in their recommended management based on clinical review of their case at the Multidisciplinary Pancreatic Cancer Clinic. Dr. Pawlik and colleagues concluded that the single-day pancreatic multidisciplinary clinic provides a comprehensive and coordinated evaluation of patients that leads to changes in therapeutic recommendations in close to one-quarter of patients.

New IPMN FAQ Page Added
- June 2008
We have added a new frequently asked questions page to the Patient Education section of this site. It's an FAQ on a type of precancerous lesion called the Intraductal Papillary Mucinous Neoplasm, or IPMN. We hope that it's helpful!


Matt Dallek's 60-Mile Bike Ride for Pancreatic Cancer Research
- May 2008
This fall, a remarkable young man named Matt Dallek will go on a 60-mile bike ride through the civil war battlefields of Maryland to raise money for pancreatic cancer research at Johns Hopkins. Matt is wonderful, thoughtful and brave. You can learn more about Matt's remarkable story and read the e-mail Matt sent out announcing his ride by clicking here.

Randy Pausch’s Journey with Pancreatic Cancer
- April 2008
Many of you may have heard about Randy Pausch, a computer science professor at Carnegie Mellon University and father of three who has been diagnosed with pancreatic cancer. Last September, Randy gave an inspirational “Last Lecture” at Carnegie Mellon entitled “Really Achieving Your Childhood Dreams.” Since that time, Randy’s lecture has garnered worldwide attention from both the public and the media, with appearances on Oprah Winfrey and Diane Sawyer. Importantly, on March 13, 2008, Randy testified before a US Senate subcommittee, advocating for greater federal funding for pancreatic cancer. Randy’s story is a testimony to the power of courage in the face of difficult battle: pancreatic cancer.
A number of you have asked for more information about Randy. We therefore thought it might be helpful to provide some key links.
Watch The Last Lecture here:
https://www.cmu.edu/randyslecture/
Visit Randy’s Story
https://www.cmu.edu/randyslecture/story/index.html
One way you too can help us fight this disease is by participating in research studies such as the NFPTR. To learn more, please click here.


Congratulations Dr. Anirban Maitra!
- March 2008
On March 4th, 2008 Dr. Anirban Maitra, Associate Professor of Pathology and member of the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins, was awarded the Ramzi Cotran Young Investigator Award from the United States and Canadian Academy of Pathology. This award was presented at the Academy's annual meeting in Denver. The Young Investigator Award recognizes a body of work which has contributed significantly to the diagnosis and understanding of human disease. Dr. Maitra received this important award because of his ground-breaking work on pancreatic cancer. A complete description of the award can be found on the Academy's web site (https://www.uscap.org) and then go to 2008 Annual Meeting and Program Book, then click on Ramzi Cotran Young Investigator Award - Anirban Maitra. Congratulations Dr. Maitra!
2007 ▼
5th Annual RUN FOR GEORGE Update
- December 17, 2007
The family of George Rubis visited the pancreatic cancer research team at Johns Hopkins on Thursday December 13th, 2007. he family toured the labs and enjoyed a break with team members (click here to see photo). The family presented Dr. Iacobuzio-Doanhue a check for over $70,000 from the 5th Annual Run for George to support her pancreatic cancer research efforts! Congratulations to the Rubis Family!
View More Pictures

Golf Classic
- September 17, 2007
The Joseph C. Monastra Foundation for Pancreatic Cancer Research will hold a Golf Tournament to raise monies to support the pancreatic cancer research efforts of Dr. Christine Iacobuzio-Donahue here at Johns Hopkins. This "Golf Classic" will be held on October 29, 2007 in Alpharetta, Georgia. Contact Rick Rosemond at 678-888-0243 for more information.

Dr. Joseph Herman interviewed on satellite radio
- September 4, 2007
Dr. Joseph Herman, co-founder of the Johns Hopkins Multi-Disciplinary Pancreatic Cancer Clinic and an Assistant Professor of Radiation Oncology & Molecular Radiation Sciences was recently interviewed on ReachMD XM 157, an XM radio station for medical professionals. Dr Herman discussed some of the unique features on diagnosing pancreatic cancer that are very helpful for patients/family members. He also talked about the newer options in terms of treating pancreatic cancer and also the benefits of the Johns Hopkins Multi-Disciplinary Clinic.
To listen, click a link below. To save the file to your computer, right click and choose "save":
Interview #1
Interview #2

Dr. Ralph Hruban interviewed on satellite radio
- July 2007
Dr. Ralph Hruban, Director of the Sol Goldman Pancreatic Cancer research Center here at Johns Hopkins was recently interviewed on ReachMD XM 157, an XM radio station for medical professionals. In a series of three interviews Dr. Hruban discussed the current state of our understanding of pancreatic cancer. Although these interviews are geared towards medical professionals, we thought you might find them educational.
To listen, click a link below. To save the file to your computer, right click and choose "save":
Interview #1
Interview #2
Interview #3

Rolfe Foundation's Support Tops $1 Million
- May 2007
The Michael Rolfe Pancreatic Cancer foundation has been a major supporter of pancreatic cancer research at Johns Hopkins. Over the last 8 years this remarkable foundation has given over $1 Million to support research aimed at the early detection of pancreatic cancer. These funds have provided a critical boost to our early detection efforts, they have helped a number of young talented physician-scientists join the pancreatic cancer research team, and they have provided funds for our scientists to obtain the preliminary data needed to get larger grants from the Federal government. Most importantly, the work of the Rolfe Foundation demonstrates how one family and their dedicated friends can make a significant difference in the war against pancreatic cancer. To learn more about Michael Rolfe and the Michael Rolfe Pancreatic Cancer Foundation please visit their web site.

Congratulations Dr. Elliot Fishman
- May 2007
Dr. Elliot Fishman here at Johns Hopkins was named the best radiologist in the United States in a survey of other radiologist by Medical Imaging magazine. Dr. Fishman is a Professor of Radiology and Oncology, and an active participant in the Johns Hopkins Multi-Disciplinary Pancreatic Cancer Clinic. Dr. Fishman has published extensively on CT imaging of pancreatic neoplasms and has a highly successful educational Web site. This web site includes the interactive "Ask the Fish" module. Congratulations Dr. Fishman!

Congratulations Dr. Sydney Dy
- April 2007
We are pleased to announce that Sydney Dy, M.D., M.Sc., has been named director of the Johns Hopkins Cancer Center's Harry J. Duffy Pain and Palliative Care Program. The new program has been developed through the generosity of the Duffy family to benefit all patients at the Johns Hopkins Kimmel Cancer Center.
As a member of the Hopkins faculty since 2001, Sydney holds joint appointments in the Bloomberg School of Public Health Division of Health Policy Management as well as in the School of Medicine Department of Medicine and Oncology. Sydney is board certified in hospice and palliative medicine and has extensive experience in palliative care quality standards and quality improvement. She currently serves on the NCCN's palliative care clinical practice guidelines committee, and is part of a national project to develop pilot supportive oncology quality indicators. Her expertise in research and clinical practice bring together two important goals for this program.
The pain and palliative care team will provide support with comprehensive symptom management, coordination of care, and assist patients and families with decision-making. Initially, the service will be piloting on one of the inpatient units (Weinberg 5D), and then available to all Cancer Center patients within the next several months. Sydney and a multidisciplinary team of nurses, social workers, and others including pharmacy, nutrition and rehabilitation medicine, will coordinate with faculty and staff on Weinberg oncology inpatient units during and after rounds, and with outpatient clinicians to address patient and family needs.
Lynn Billing, B.S.N.,C.H.P.N., is the program's nurse coordinator.
Please join us in welcoming Sydney to her new role.

New Tool for Risk Assessment for Individuals With a Family History of Pancreatic Cancer
- April 2007
Dr. Alison Klein and colleagues at Johns Hopkins have recently developed a clinical tool, "PancPRO" to help estimate an individuals risk of developing pancreatic cancer based upon their family history of pancreatic cancer. This computer model will help identify individuals with an increased risk of developing pancreatic cancer who may want to pursue early detection screening or genetic counseling. This model was developed and validated using information provided by the families enrolled in the National Familial Pancreas Tumor Registry and provides a better assessment of risk than just counting the number of family members with pancreatic cancer because it looks at the entire family tree and the age-of-onset of pancreatic cancer in affected family members. This model estimates the probability that an individual carries a putative pancreatic cancer susceptibility gene and based upon this risk estimates the lifetime risk of developing pancreatic cancer.
PancPRO is maintained as part of the Hopkins SPORE in Gastrointestinal Cancer with Alison Klein and Giovanni Parmigiani as project leaders. It is currently distributed free of charge for research and counseling use through BayesMendel or CaGene.
These results are described in the current issue of the Journal of Clinical Oncology.
PancPRO: Risk Assessment for Individuals With a Family History of Pancreatic Cancer
Wenyi Wang, Sining Chen, Kieran A. Brune, Ralph H. Hruban, Giovanni Parmigiani, and Alison P. Klein
JCO Apr 10 2007: 1417-1422.

Hopkins Pancreatic Cancer Researchers Among Most Highly Cited by Their Peers
- February 2007
Four members of the Johns Hopkins Pancreatic Cancer Team were recently recognized by Essential Science Indicators as being in the Top 10 most highly cited Pancreatic Cancer scientists in the world. In a review of scientific papers published between 1996 and October 31, 2006, Drs. Ralph Hruban, Scott Kern, Michael Goggins and John Cameron were recognized as highly cited authors. Dr. Hruban was recognized as the most highly cited of all of pancreatic cancer investigators. In addition, the paper by the Hopkins investigators reporting the discovery of the DPC4 pancreatic cancer gene is recognized by Essential Science Indicators as the most highly cited paper in all of pancreatic cancer research. An author is considered cited when another investigator specifically references ("cites") their work in a medical or scientific paper. Citations are one of many indicators of the impact of scientific work. Congratulations to the Hopkins team!
2006 ▼

Oncology on Canvas Competition
- December 2006
At an awards finale hosted by Regis ("Live with Regis and Kelly") and Joy Philbin at New York's Metropolitan Pavilion, Johns Hopkins Multi-Disciplinary Clinic Nurse coordinator JoAnn Coleman won second place in the 2006 Lilly Oncology on CanvasSM: Expressions of a Cancer Journey International Art Competition and Exhibition for her photograph, "Tranquility." The award was presented by Eli Lilly and Company in partnership with the National Coalition for Cancer Survivorship (NCCS). JoAnn Coleman has been a nurse at the Johns Hopkins Hospital's Department of Surgery since 1974 and now serves as coordinator of the Multidisciplinary Pancreatic Cancer Clinic at The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. "Tranquility" shows a serene fountain in Hawaii containing water lilies with a reflection of a Japanese Tea House. JoAnn took the photograph while in Hawaii for a nursing conference.
"The photograph reminds me of how we treat patients when they go into surgery for the removal of cancerous tumors," said Coleman. "We should accept and respect patients as they journey through the cancer experience."
Patients faced with the challenges of cancer experience the gamut of emotions that reflect the mood of the ocean from peaceful laps of waves at the shore to waves of tumultuous, rolling fury. I care for patients with pancreatic cancer and follow their journey along the continuum of care. I see the complex and ever-changing combination of physical, sensual and spiritual elements encountered by these patients and their families/significant others.
This photo on the right is of a Japanese tea house reflected in a pond of elegant purple water lilies represents my philosophy of trying to create an aura of tranquility for my patients and their families/significant others. The lovely purple water lilies are a reminder of patients who have pancreatic cancer attempting to maintain harmony but always with a ripple of the reminder of the unknown that may be encountered in their cancer journey. My patients have taught me to respect the human qualities of sensitivity, awareness, respect and communication which are also those behaviors reflected in the ancient and elegant tradition of the Japanese tea ceremony.

Report of a pancreatic cancer susceptibility gene in a unique pancreatic cancer family
- December 2006
Investigators at the Universities of Washington and Pittsburgh have recently identified an inherited gene mutation that is the likely mutation that causes pancreatic cancer susceptibility in a unique family known as Family X. Family X is a unique pancreatic cancer family because individuals in the family develop pancreatic cancer at a young age (< age 40) typically after many years of pancreatic insufficiency (inadequate production of digestive enzymes by the pancreas) as well as diabetes mellitus and extensive structural alterations of the pancreas. The mutation in this family was found in a gene known as palladin. We are currently determining if mutations in this gene are responsible for inherited pancreatic cancer in our NFPTR families. Our current evidence based on a method known as linkage analysis applied to NFPTR families and additional families in the PACGENE (pancreatic cancer genetic epidemiology) consortium indicates that our families probably do not harbor inherited mutations in the region where the palladin gene is located. Notably, none of the families enrolled in the NFPTR have the clinical features that characterize Family X. Generally, in families that have multiple relatives with pancreatic cancer, affected members develop their cancer at an older age (typically over 40) and without pancreatic insufficiency. Further studies are required to determine if acquired mutations occur in the palladin gene. Hopefully, the discovery of the causal mutation in Family X will ultimately lead to additional discoveries that can be used for other families affected by pancreatic cancer. Indeed, understanding the function of Palladin may ultimately provide further insights as to how pancreatic cancer develops. If you have further questions, please feel free to contact NFPTR coordinator Emily Palmisano at [email protected].
These results are described in the current issue of Public Library of Science, Medicine.
Reference:
Kay L. Pogue-Geile, Ru Chen, Mary P. Bronner, Tatjana Crnogorac-Jurcevic, Kara White, Sally Dowen, Carol A. Otey, David A. Crispin, Ryan D. George, David C. Whitcomb, Teresa A. Brentnall. Palladin Mutation Causes Familial Pancreatic Cancer: Uncovers a New Cancer Mechanism.

Multi-Disciplinary Pancreatic Cancer Clinic
- November 2006
We are pleased to announce the opening of the Multi-Disciplinary Pancreatic Cancer Clinic at The Johns Hopkins Hospital.
The clinic will be held every Tuesday, and the first clinic day will be November 21st, 2006. In one day patients with known or presumed pancreatic cancer will have a comprehensive evaluation by nationally recognized pancreatic cancer clinicians and specialists.
It is our goal that all patients will be seen in the clinic within one week of the referral, as long as all the necessary clinical information has been obtained.
The Multi-Disciplinary Pancreatic Cancer Clinic is designed to evaluate patients with known or suspected pancreatic cancer. The clinic is committed to a comprehensive single day evaluation incorporating all the resources available for the education, diagnosis, treatment and research of pancreatic cancer.
A patient will:
1. Have access to the following specialists:
- Medical oncology
- Radiation oncology
- Surgical oncology
- Gastroenterology
- Pathology
- Radiology
- Genetics counseling
- National Familial Pancreas Tumor Registry
- Nutrition
- Clinical trials
- Oncology Nurses
- Pastoral care
- Pain management
- Social Work
2. Have a recommended plan of treatment provided in writing at the end of the visit
3. Rapid reports to the patient's primary care and/or referring physician.
To make an appointment at the Multi-Disciplinary Pancreatic Cancer Clinic, please call 410-933-PANC (410-933-7262).

Dr. Ralph Hruban awarded the 2006 PanCAN Medical Visionary Award
- November 2006
Ralph H. Hruban, M.D., Director of the Sol Goldman Pancreatic Cancer Research Center at The Johns Hopkins University School of Medicine, was awarded the 2006 Medical Visionary Award from PanCAN, the national pancreatic cancer patient advocacy group. The Medical Visionary Award honors a prominent individual in the medical or scientific community whose outstanding achievements advance the cause of pancreatic cancer research. Dr. Hruban received this award at PanCAN's annual "Evening with the Stars" gala in Hollywood California on November 4, 2006. Nancy Daly Riordan, wife of the former Mayor of Los Angeles Richard Riordan, was also honored at the gala with the Emily Couric Public Service Award.



More photos courtesy of Marsha Garil - Evening with the Stars and Friday Night Reception



Mouse Tests Predict Drug Response In Relapsing Pancreatic Cancer Patients
- October 2006
Dr. Antonio Jimeno, M.D., instructor in Oncology at the Sol Goldman Pancreatic Cancer Research Center reported in a recent issue of Clinical Cancer Research (Pub Med PMID: 16899615) a new approach to individualizing therapy for patients with pancreatic cancer. By slicing up bits of patient tumors and grafting them into mice, Dr. Jimeno has figured out how to accurately "test drive" chemotherapy drugs to learn in advance which drug treatments offer each individual pancreatic cancer patient the best therapeutic journey.
Although "xenografting" with either cells or fresh tissue is already used widely to study cancer in the lab, the Hopkins design is personalized to each patient who has relapsed after an initial course of chemotherapy. "Eventually our approach offers a promising way to individualize therapy earlier in treatment instead of first giving everyone the standard drug gemcitabine, which has a success rate of less than 10 percent," says Dr. Jimeno, M.D.

PanCan Visit
- September 2006
On Saturday, September 9, 2006, 24 members of the Washington DC PanCAN group visited The Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins. The visit was organized by Mary Zapor and Marsha Garil from PanCAN. The PanCANvisitors toured some of the labs and listened to talks on the ongoing pancreatic cancer research and clinical treatment of pancreatic cancer at Johns Hopkins. After rotating through the labs, the group had an informal lunch with the doctors and scientists. Three members of the PanCAN group that had visited in 2003 returned to visit the new location in the Cancer Research Building II. A number of people in the group commented on how informative the visit was, and several of the doctors and scientists said that meeting patients and family members provides an inspiration for their work. Below are pictures from this wonderful visit which demonstrates the value of patient advocates, physicians and scientists working together.

















Dr. Cameron on Baltimore Sun
- August 2006
The Baltimore Sun has a two-part article on Johns Hopkins pancreatic cancer surgeon Dr. John Cameron. The article can be found on The Baltimore Sun. Part one describes Dr. Cameron's surgery and part two describes the impact his work has had forming a basis for the Johns Hopkins pancreatic cancer research team. Additional audio and photos are also available. Enjoy!


Dr. Elizabeth Jaffee Wins Award
- July 2006
Congratulations go out to Dr. Elizabeth Jaffee. Dr. Jaffee was awarded the Outstanding SPORE Investigator today at the annual meeting of the National Cancer Institutes SPORE (Specialized Program of Research Excellence) investigators meeting. The award, "in recognition of pursuit of excellence in translational research," was given to Dr. Jaffee for her work developing the vaccine treatment for pancreatic cancer. Dr. Jaffee is a Professor of Pathology and Oncology at Johns Hopkins.

Johns Hopkins Is Number One Again!!!
- July 2006
THE JOHNS HOPKINS HOSPITAL TOPS U.S.NEWS & WORLD REPORT'S "HONOR ROLL" FOR THE 16th YEAR IN A ROW
The Johns Hopkins Hospital has again earned the top spot as "Best of the Best" in U.S. News & World Report's annual Honor Roll of American hospitals, placing first in five of 16 ranked medical specialties and in the top four in 10 others. Only 14 hospitals nationwide made it to the Honor Roll this year out of 5,189 institutions graded.
This year's annual guide reports results of a survey of a hospital's reputation among a national sample of physicians, along with analysis of objective indicators such as death rates, technology, nurse staffing, service mix and discharge planning.
For a detailed list of all rankings, go to www.hopkinsmedicine.org or to the U.S. News and World Report.

Hopkins Researcher Wins Maryland Outstanding Young Scientist Award
- June 2006
Anirban Maitra, Associate Professor of Pathology and Oncology at The Sol Goldman Pancreatic Cancer Research Center has been awarded the Maryland 2006 Outstanding Young Scientist Award. The award is sponsored by the Maryland Academy of Sciences and conferred by the Maryland Science Center in the hopes of encouraging the important work of young scientists in the state of Maryland, and to increase public awareness of their accomplishments.
The Maryland Outstanding Young Scientist award program was established in 1959 to recognize and celebrate extraordinary contributions of young scientists in Maryland. Many previous recipients of these awards have gone on to distinguished careers in science including one recipient who is now a Nobel laureate.
Along with a monetary award, the Maryland Outstanding Young Scientist receives the Allan C. Davis medal. Mr. Davis was a distinguished chairman of the board from 1959 until1966, and a gift from his family allowed the Science Center to build the Davis Planetarium as part of the current Maryland Science Center that opened at the current location in 1976.
The award had been given annually until 1998 when the program was suspended while the Maryland Science Center entered into a strategic planning and capital expansion program. Now, as we mark the Science Center's 30th anniversary, it is only fitting to return the award to its annual status.
While it is the accomplishments of the Maryland Outstanding Young Scientist that ultimately decide which nominee is given the award, there are two other qualifications that must be met: He or she must be a Maryland resident and he or she can be no older than 35. The nominees are reviewed and discussed at length by judges from our Scientific and Educational Advisory Council and it is their group who chooses the award winner.
Dr. Maitra's research has led to several new potential treatments for pancreatic cancer. His work focuses on genetics since pancreatic cancer is fundamentally a genetic disease. His research has provided key insights into the mechanisms underlying the genetic changes that characterize pancreatic cancer. He then develops new therapies which exploit these changes to target pancreatic cancer cells selectively, without harming normal cells. His award winning writings on his work have been published in Nature, The American Journal of Pathology, Genome Research, and many other noteworthy journals.
Full script: Good Evening and Welcome to the Maryland Science Center-I'm Van Reiner, President and CEO of the Maryland Science Center and we're here tonight to award the Allan C. Davis medal to Maryland's Outstanding Young Scientist. The Maryland Outstanding Young Scientist award program was established in 1959 to recognize and celebrate extraordinary contributions of young scientists in Maryland. Many previous recipients of these awards have gone on to distinguished careers in science including one recipient who is now a Nobel laureate.
Along with a monetary award, the Maryland Science Center Outstanding Young Scientist receives the Allan C. Davis medal. Mr. Davis was a distinguished chairman of the board from 1959 until1966 and a gift from his family allowed us to build the Davis Planetarium as part of the current Maryland Science Center that opened at this location in 1976. The award had been given annually until 1998 when the program was suspended while the Maryland Science Center entered into a strategic planning and capital expansion program. Now, as we mark the Science Center's 30th anniversary here at its Inner Harbor location it is only fitting to return the award to its annual status.
While it is the accomplishments of the Maryland Outstanding Young Scientist that ultimately decide which nominee is given the award, there are two other qualifications that must be met: He or She must be a Maryland resident and he or she can be no older than 35. The nominees are reviewed and discussed at length by judges from our Scientific and Educational Advisory Council and it is their group who chooses the award winner. Our Scientific and Advisory Council is headed by Dr. Robert Cotter who is a professor in the department of pharmacology and molecular sciences at the Johns Hopkins University School of Medicine. The council is a policy and programming advisory group that helps the Maryland Science Center review its exhibits and programs, and their content, for accuracy and educational value. The Scientific Council is composed of scientists, researchers, teachers, physicians, engineers and other professionals from the fields of science, education, and technology. In just a few moments I'll ask Dr. Cotter, who is with us tonight, to join me for the award presentation.
Tonight's recipient of the Maryland Outstanding Young Scientist award and Allan C. Davis medal is an Associate Professor of Pathology and Oncology at the Johns Hopkins University School of Medicine. He was born in 1972 and lives in Kensington Maryland. He has published 139 peer-reviewed publications, 12 invited reviews, and 22 book chapters. He has 7 provisional patents or notices of invention and holds 6 active grants from the National Institutes of Health. He teaches second year medical students, graduate students, residents, and fellows at Johns Hopkins. In his nomination letter it was said that perhaps the most remarkable thing about this year's recipient is that he is "one of the world's nicest human beings-in an age of cutthroat competition he is extraordinarily collaborative." Tonight's Outstanding Young Scientist is also a gifted researcher who focuses on one of the most difficult diseases of all diseases to study-aggressive forms of cancer and pancreatic cancer specifically. It is estimated that 1 in every 5000 Marylanders develops cancer each year and Maryland owns the sixth highest cancer death rate in the nation.
Tonight's awardee's research has led to several new potential treatments for pancreatic cancer. His work focuses on genetics since pancreatic cancer is fundamentally a genetic disease. His research has provided key insights into the mechanisms underlying the genetic changes that characterize pancreatic cancer. He then develops new therapies which exploit these changes to target pancreatic cancer cells selectively, without harming normal cells. His award winning writings on his work have been published in Nature, The American Journal of Pathology, Genome Research, and many other noteworthy journals. Clearly our award recipient's research and scholarship qualifies him for the award. His work is truly groundbreaking and the respect and support of his peers cannot be overlooked. And his insistence on collaboration is extremely noteworthy. In short, he is the ideal recipient of the Maryland Outstanding Young Scientist Award.
Please join me in saluting this year's recipient of the Allan C. Davis Medal, Dr. Anirban Maitra-this year's Maryland Outstanding Young Scientist.

Animation Illustrating How Precursor Lesions in the Pancreas Produce Changes in the Pancreas that can be Detected by Endoscopic Ultrasound
- May 2006
Lauren O'Malley, a student in the Division of Art as Applied to Medicine at Johns Hopkins recently completed a unique animation as a part of her Masters thesis. This animation beautifully illustrates the changes that occur in the pancreas before an invasive cancer develops, and how these changes can be detected by endoscopic ultrasound (see the May 11, 2006 What's New below). Ms. O'Malley's work was supported by a generous grant from the Vesalius Trust. We hope you find this animation educational.

Screening for Early Pancreatic Neoplasia in High-Risk Individuals
- May 2006
Dr. Marcia Canto and colleagues from Johns Hopkins report the results of the “CAPS 2” screening for early pancreatic cancer program in the June issue of Clinical Gastroenterology and Hepatology (link to PMID: 16682259). Dr. Canto screened 72 individuals with a strong family history of pancreatic cancer, 6 patients with the Peutz-Jeghers Syndrome, and 149 controls using a combination of endoscopic ultrasound (EUS) and computerized tomography (CT scanning). If something abnormal was identified the patients also underwent endoscopic retrograde cholangiopancreatography (ERCP). All of the patients were asymptomatic. Remarkably, 8 of the patients were found to have a tumor in their pancreas (10% yield of screening); 6 patients had 8 benign intraductal papillary mucinous neoplasms (IPMNs), 1 had an IPMN that progressed to invasive ductal adenocarcinoma, and 1 had pancreatic intraepithelial neoplasia. Endoscopic ultrasound (EUS) and computerized tomography (CT scanning) also diagnosed 3 patients with 5 extrapancreatic neoplasms. Endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography abnormalities suggestive of chronic pancreatitis were more common in high-risk patients than in control subjects. From these studies, Dr. Canto concluded that screening EUS and CT diagnosed significant asymptomatic pancreatic and extrapancreatic neoplasms in high-risk individuals (people with a strong family history of pancreatic cancer and people with the Peutz-Jeghers Syndrome). Dr. Canto also concluded that abnormalities suggestive of chronic pancreatitis are identified more commonly in high-risk individuals. Dr. Canto plans to start “CAPS 3.” More information about this research screening protocol will be posted on this web site as it becomes available.
Reference:
Clin Gastroenterol Hepatol. 2006 May 5.

DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease
- March 2006
The biggest challenge of pancreatic cancer is to try to detect the cancer at an early stage when it can be surgically removed. Dr. Michael Goggins’ Early Detection Laboratory is dedicated to identifying new markers for the early detection of pancreatic cancer. Just as there is mammography for breast cancer and the PSA test for prostate cancer, so too do we need a test for early pancreatic cancer. Scientists working in The Early Detection Laboratory at Johns Hopkins examined the DNA in pancreatic juice samples that were collected from pancreatic cancer patients to determine if the DNA from these samples showed an abnormal amount of methylation. Methylation is the addition of an additional carbon to specific areas of the DNA. Hypermethylation (or too much methylation) of certain genes will stop the genes from working properly. In particular, hypermethylation of genes whose normal function is to protect a cell from developing into a cancer (tumor suppressor genes) or whose function is to repair damaged DNA (mismatch repair genes) have been associated with the development of cancer. The scientists found that there was more methylation in the pancreatic juice samples collected from pancreatic cancer patients than there was in the samples collected from chronic pancreatitis patients and patients with no history of pancreatic disease. This finding is important because it suggests that the detection and quantification of methylated DNA in pancreatic juice may provide a promising approach to the diagnosis of pancreatic cancer.
Reference:
Matsubayashi H, Canto M, Sato N, Klein A, Abe T, Yamashita K, Yeo CJ, Kalloo A, Hruban R, Goggins M. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006 Jan 15;66(2):1208-17.

The effect of a Frequently Asked Questions module on a Pancreatic Cancer Web site patient/family chat room
- February 2006
When a person is diagnosed with pancreatic cancer, the web can be a powerful resource to gather information. Patients often turn to the web to gather information that will help them to make decisions regarding their treatment and to find support from other individuals who are experiencing similar issues. It is therefore imperative that the information provided is accurate, easy to find, and that it addresses the questions patients have. Joanne Coleman R.N. at Johns Hopkins and her colleagues looked at the posts on our discussion board to determine if the “Illustrated Frequently Asked Questions” (FAQ) section of the Pancreatic Cancer Webpage was helpful to patients and caregivers who were seeking information about this cancer. The group found that the FAQ section was helpful to individuals who wanted more information about this disease. She also identified ways in which the FAQ section could be improved. More information was needed on pain management and care giving during the final stages of the disease. This information has since been added to our FAQ section. Ms. Coleman’s study was recently published in Cancer Nursing. It is our hope that the Johns Hopkins Pancreatic Cancer Webpage provides important and useful health information and that our discussion board provides a supportive environment for patients and caregivers. If you have any suggestions for topics that you would like addressed in our FAQ section, please send us an email via the feedback link on the discussion board.
Reference:
Coleman J, Olsen SJ, Sauter PK, Baker D, Hodgin MB, Stanfield C, Emerling A, Hruban RH, Nolan MT. The effect of a Frequently Asked Questions module on a pancreatic cancer Web site patient/family chat room. Cancer Nurs. 2005 Nov-Dec;28(6):460-8.

A Fish Oil Supplement to Maintain Body Weight in Patients With Cancer
- January 2006
Protocol Title: AAFA Fish Oil Nutritional Supplementation in Patients with Disease Related Weight Loss
Protocol Number: J0275
Type of Cancer: Multiple: Solid Tumors
Physician: Adrian Dobs
Phase Number: II General
Eligibility: Diagnosis of cancer, except esophageal cancer. 18 years of age and older. Must be 6 weeks after surgery. Concurrent chemotherapy or radiation therapy is okay. Good blood counts and kidney function. No use of fish oil supplements in the past 3 months. No vitamin usage greater than 150% RDA. No use of medicinal herbs in past month. No HIV or hepatitis. Women must not be pregnant or breastfeeding.
Purpose: The overall goal of this project is to evaluate the safety and efficacy of nutritional supplementation with fish oil in the maintenance of weight in cancer patients. Patients with some cancers have a high occurrence of cancer cachexia with resultant weight loss and since mainstream treatment does not sustain weight, fish oil supplementation will be tested in this trial because there is data to suggest that fish oil can affect the underlying inflammatory response that leads to weight loss.
Treatment: Qualified individuals are treated with fish oil supplements or placebo. The fish oil is packaged in the form of small tubes of oil that can be added to either juice or sports drinks or liquid nutritional supplements. Treatment is for 12 weeks. The study has 5 visits.
Please contact Anne Munson: [email protected]
2005 ▼
Pancreatic Cancer in Individuals of Ashkenazi Jewish Ancestry
- December 2005
One of the first steps to effective screening for early pancreatic cancer is the identification of individuals with an increased risk of developing the disease. For years it has been known that individuals of Ashkenazi Jewish descent have an increased risk of developing pancreatic cancer, but the basis for this increased risk is only now being elucidated. Inherited mutations (DNA changes) in the first and second breast cancer genes, called BRCA1 and BRCA2, explain some of this increased risk. In order to provide more details about this increased risk we have added a new section to this web site . This new section is entitled "Pancreatic Cancer in Individuals of Ashkenazi Jewish Ancestry". We hope you find this information educational and useful.

Early Results Using Therapeutic Pancreatic Cancer Vaccine Show Promise
- November 2005
Researchers in the Sol Goldman Pancreatic Cancer Research Center in the Johns Hopkins Kimmel Cancer Center are encouraged by early results of a treatment vaccine for pancreatic cancer. At about two years into a study of 60 patients, the researchers report that 88 percent survived one year and 76 percent are alive after two years.
"Even though our results are preliminary, the survival rates are an improvement over most published results of pancreatic cancer treatment studies," says Daniel Laheru, assistant professor at the Johns Hopkins Kimmel Cancer Center. Laheru is expected to present his findings in a press briefing at a joint meeting of the American Association for Cancer Research/National Cancer Institute/European Organization for Research and Treatment of Cancer in Philadelphia on November 15.
Until recently, most studies have shown pancreatic cancer survival rates at about 63 percent one year after diagnosis and 42 percent at two years. The long-term outlook is more grim - only 15 to 20 percent of patients with local disease are alive at five years. "Since there is no universal standard for treating pancreatic cancer, it is difficult to make direct comparisons between all the studies, " says Laheru.
In the current study, his team combined an immune-boosting vaccine with surgery and conventional postoperative chemotherapy and radiation. The vaccine, originally developed at Johns Hopkins, uses irradiated pancreatic cancer cells incapable of growing, but genetically altered to secrete a molecule called GM-CSF. The molecule acts as a lure to attract immune system cells to the site of the tumor vaccine where they encounter antigens on the surface of the irradiated cells. Then, these newly armed immune cells patrol the rest of the patient's body to destroy remaining circulating pancreatic cancer cells with the same antigen profile.
Patients get one vaccine injection eight to ten weeks after surgery, then four booster shots after chemotherapy and radiation. Laheru and his team completed enrolling patients in the study this past January. The average follow-up time is 32 months.
"It is important that we continue to follow these patients to know how the treatment will work in the long-term," says Elizabeth Jaffee, M.D., the Dana and Albert "Cubby" Broccoli Professor in Oncology and Pathology. "We're hopeful that these early results will hold true."
Jaffee and Laheru hope to begin multi-institutional studies in about a year. They are working with Hopkins pathologists from the Sol Goldman Pancreatic Cancer Research Center to analyze proteins from pancreatic cancer cells that may help them refine the vaccine's targets.
This research was funded by the National Cancer Institute and in part by Cell Genesys, Inc. (see below information on the licensing agreement between the Johns Hopkins University and Cell Genesys, Inc.) and presented at the Proceedings, AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, November 2005. (Abstract #2229, A Safety and Efficacy Trial of Lethally Irradiated Allogeneic Pancreatic Tumor Cells Transfected with the GM-CSF Gene in Combination with Adjuvant Chemoradiotherapy for the Treatment of Adenocarcinoma of the Pancreas).
Questions and Answers
Is the Hopkins pancreatic cancer vaccine available now?
No, the vaccine is not commercially available. The current Phase II clinical trial of 60 patients at Johns Hopkins is closed to accrual. No additional patients can be enrolled in this study. When this study concludes, in about a year, more results will be made known. The current study is one step in the process of evaluating new therapies. Johns Hopkins researchers are working as fast as they can to develop new therapies, but it may take more than a year before the next phase of clinical studies begins at Johns Hopkins and other universities. There are no other universities currently participating in this vaccine study.
Is the vaccine available through a compassionate use program?
It is our understanding that the manufacturer of the vaccine, Cell Genesys, Inc. does not have a compassionate use program; however, you may want to check their web site for more information.
When will the next study begin? Can I put my name on a list?
It may be more than a year before the next study begins; therefore, we are unable to keep a list of individuals interested in the vaccine.
Are there other new therapies that might be helpful to me?
Research on a wide variety of pancreatic cancer treatment strategies is ongoing nationwide. Information on Johns Hopkins clinical trials, search our online list. Information about clinical trials at other cancer centers is available through the National Cancer Institute at www.cancer.gov and 1-800-4-CANCER.
How do I make an appointment with a Johns Hopkins expert?
If you would like to schedule an appointment with a Johns Hopkins pancreatic cancer specialist to learn of other treatment recommendations, please call:
Surgery: 410-955-5800
Medical Oncology: 410-955-8964
(Chemotherapy and other options)
Please note: due to the high level of interest in pancreatic cancer vaccine research at Johns Hopkins, our experts cannot answer individual phone calls or emails.
My cancer is widely metastatic. Will the vaccine be able to help me?
Our investigators do not have clear answers on whether the vaccine will be helpful to patients w ith metastatic disease, so they will be opening a study at Johns Hopkins in the next few months using the vaccine with a targeted therapy called erbitux for patients with metastatic pancreas cancer. The clinical trials section of our web site will have study information when the trial opens.
Under a licensing agreement between the Johns Hopkins University and Cell Genesys, Inc., the University is entitled to a share of royalty received by the University on sales of technology used in the study described in this news release. This arrangement is being managed by the University in accordance with its conflict of interest policies.

New Leadership in Pancreatic Cancer Surgery at Johns Hopkins
- August 2005
Dr. Charles Yeo has accepted the position of Chairman of the Department of Surgery at Thomas Jefferson University in Philadelphia, PA. While we are pleased for Dr. Yeo's promotion, we will miss him here at Hopkins.
With Dr. Yeo's departure, we are proud to announce that Dr. Kurtis Campbell has been chosen to provide continued leadership to the surgical efforts against pancreatic cancer at The Johns Hopkins Hospital. Dr. Campbell is an Associate Professor of Surgery and active attending surgeon at The Johns Hopkins Medical Institutions. He graduated from The Johns Hopkins University School of Medicine in 1986. After completing his surgical residency at Johns Hopkins, he spent an advanced year of training in pancreatic and biliary tract surgery. Thereafter, he was recruited to the Hopkins surgical faculty and has been an important part of the efforts against pancreatic cancer since that time.

Microarray Sequencing of Mitochondrial Genome Uncovers New Clues for Cancer Detection
- July 2005
* Reprinted with permission from July Issue of the Affymetrix Microarry Bulletin
Scientists led by Dr. Anirban Maitra, assistant professor of pathology, genetic medicine and oncology at Johns Hopkins Medical Center are using a new microarray-based method to rapidly sequence the genome of human mitochondria to find mutations linked to cancers, including prostate and pancreatic cancer. The microarray, called the MitoChip, sequences more than 12,000 bases of the mitochondrial genome, and takes less than a third the time of conventional sequencing methodologies.
“Right now we're using the MitoChip to look at a larger number of pancreatic tumor and juice samples to see if we are able to detect tumor-specific mutationsin pancreatic juice samples,” said Maitra. “While we developed the MitoChip to study cancer biomarkers, it certainly has uses in non-cancer applications as well, everything from evolutionary studies to metabolic diseases to aging.”
Maitra recently spoke with Amanda Baumann, a pre-doctoral fellow in Duke University's department of Pharmacology and Cancer Biology regarding the new challenges facing mitochondrial research now that the genome sequence can be readily accessed. The two discussed:
- The various types of mitochondrial mutations linked to cancer and models available for validation
- The “mass advantage” of mitochondrial genetics and the associated difficulties of distinguishing heteroplasmies
- The sensitivity, sample preparation, and genome coverage of microarray-based resequencing technology
To read the whole article, please click here.

Reaction of the Normal Pancreas to an Adjacent Pancreatic Cancer Provides Clues about Tissue Invasion and Detection
- July 2005
Two papers from the Hopkins group help define the genes that are expressed (made) in the tissues adjacent to pancreatic cancer. In the first paper by Ricci and colleagues, the technique of in situ hybridization was used to determine the genes that are turned on in the body's response (called a stromal reaction) to an infiltrating cancer (analogous to the body trying to heal a wound). Importantly, Dr. Ricci found that the genes that are made in this stromal response to an invasive pancreatic cancer are related to how aggressively the tumor invades the surrounding pancreas, and not to the underlying biology of the tumor. Understanding how pancreatic cancers invade the normal pancreas and spread to other organs is a critical step to understanding how to interfere with this process. In the second paper by Fukushima and colleagues, gene expression profiling was used to better understand the gene expression patterns of pancreatic tissue adjacent to infiltrating pancreatic cancers as compared to pancreatic tissue adjacent to chronic pancreatitis. Dr. Fukushima found 20 different genes were overexpressed in pancreatic tissue adjacent to an invasive cancer compared to normal pancreatic tissue adjacent to chronic pancreatitis. These results demonstrate that some of the molecular alterations in normal pancreatic tissues that occur in response to adjacent infiltrating pancreatic ductal adenocarcinoma can provide a rich source of markers for detecting pancreatic cancer.
References:
Ricci F, Kern SE, Hruban RH, Iacobuzio-Donahue CA. Stromal Responses to Carcinomas of the Pancreas: Juxtatumoral Gene Expression Conforms to the Infiltrating Pattern and Not the Biologic Subtype. Cancer Biol Ther. 2005 Mar 23;4(3)
Fukushima N, Koopmann J, Sato N, Prasad N, Carvalho R, Leach SD, Hruban RH, Goggins M. Gene expression alterations in the non-neoplastic parenchyma adjacent to infiltrating pancreatic ductal adenocarcinoma. Mod Pathol. 2005 Jun;18(6):779-87

Gastrointestinal Cancer Rapid Medical Donation Program for Pancreatic Cancer
- July 2005
In February 2003, Dr. Christine Iacobuzio-Donahue and colleagues at Johns Hopkins initiated a new program for patients with advanced stage pancreatic cancer, called the Gastrointestinal Cancer Rapid Medical Donation Program, or GICRMDP. Through this program, patients with pancreatic cancers that have spread outside the pancreas to other organs can consent to have a research autopsy. Through the hard work and team effort approach at Johns Hopkins, this program has grown to the largest of its kind in the country with patients from more than 13 different states participating as of June 2005. The tissues collected through this program are now providing an invaluable resource for ongoing research of the causes and treatment of pancreatic cancer at Johns Hopkins. Initial findings related to the tissues collected through this program have been published recently in the journal Cancer Biology and Therapy. In this paper, Dr. Iacobuzio-Donahue showed that deletions of key genes are present at a higher rate in advanced stage pancreatic cancers than previously recognized and provides the first clues to why pancreatic cancers may spread to other organs.
Reference:
E.E. Embuscado, D. Laheru, F. Ricci, K.J. Yun, S. Witzel, A. Seigel, K. Flickinger, M. Hidalgo, G.S. Bova, C. A. Iacobuzio-Donahue. Immortalizing the Complexity of Cancer Metastasis: Genetic Features of Lethal Metastatic Pancreatic Cancer Obtained from Rapid Autopsy. Cancer Biol. Ther. 2005 May 12;4(5)

Shaarey Zedek Kindergarten
- July 2005
We recently received the following moving letter and donation from a very talented Kindergarten class.
Dr. Hruban,
As our Kindergarten year ends once again this year. I am happy to say that my students created a wonderful Britto canvas and auctioned it off at our "Gallery Opening Night." This year, our 10 boys (yes we had all boys this year) raised $190 for Pancreatic Cancer Research in memory of my Mom, Rebecca Nathanson. This is our 6th year doing this activity. Please find a cure for Pancreatic Cancer quickly. I hate to think of all of the people suffering with this horrible disease like my Mom did.
Best wishes,
Shelly Mettzer
Shaarey Zedek Kindergarten
West Bloomfield, Michigan


Precursor Lesions of Pancreatic Cancer Catching the Horse before It has Fled the barn
- July 2005
Three recent articles by Johns Hopkins scientists have expanded our knowledge of the precursor lesions which are thought to develop into invasive pancreatic cancers. Understanding the biology of these precursor lesions is of critical importance if we are to detect, and potentially treat, pancreatic cancer before it spreads. In the first article, Anirban Maitra and colleagues comprehensively reviewed the various subtypes of precursor lesions that are known to progress to invasive pancreatic cancer. While Pancreatic Intraepithelial Neoplasia or "PanIN" is the prototype precursor lesion associated with the "usual" cancers (ductal adenocarcinomas), other larger precursor subtypes such as intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) are being increasingly recognized with better imaging and screening techniques. The pancreatic cancers that arise in the context of these latter precursor lesions, particularly IPMNs, can have a different biology and outcome than the usual PanIN-associated invasive cancers. Therefore, it is important that pathologists who examine surgically removed pancreata are familiar with the histology of the various non-invasive precursors to invasive pancreatic cancers.
The second and third articles identify potential therapeutic targets in PanINs, the most common precursor lesion of pancreatic cancer. Inactivation of function of a critical gene that controls the cell cycle – p16 (CDKN2A) – is extremely frequent in invasive pancreatic cancers. In about a third of these cases, loss of p16 function occurs via deletion of both p16 gene copies (called "homozygous deletion" in genetic terminology) and is large enough to include a neighboring gene known as MTAP in the deletions. Complete loss of MTAP function can be exploited for therapy using drugs that selectively affects MTAP-negative pancreatic cancer cells without damage to normal tissues. Now, Dr. Hustinx and colleagues have shown that even subsets of non-invasive precursor lesions (PanINs) harbor deletions of both copies of the MTAP gene. This is the first demonstration of a homozygous deletion in a PanIN lesion, and the authors have described a simple assay that will enable determining MTAP gene status in limited tissue materials, such as biopsy specimens. Why is this important? Compounds that selectively target MTAP-negative pancreatic cancers are already in clinical trials; if successful, one can envision extrapolating these trials to treat the precursors to invasive cancer before a cancer develops. The challenge will be in identifying patients with non-invasive, MTAP-negative precursor lesions who might potentially benefit from this therapy. With advances in molecular imaging and biopsy techniques, scientists are hopeful that this strategy will be actualized in the future.
In the third article, Dr. Prasad and colleagues performed the first large scale gene expression profiling of PanIN lesions using "gene chips". Johns Hopkins scientists were one of the first to comprehensively determine the gene expression profile of invasive pancreatic cancer, and the group at Hopkins has now extended this knowledge to precursor lesions as well. In order to isolate these tiny ductal lesions from surrounding normal tissues, the scientists used a technique called "laser capture microdissection". They found 49 genes that were expressed differentially compared to normal ductal epithelium. Of note, they found many of the overexpressed genes in PanINs are normally turned on by activation of the sonic hedgehog pathway during development, thereby confirming that abnormal activation of this pathway plays a role in both early and advanced pancreatic cancers. Hopkins scientists had previously demonstrated that the sonic hedgehog pathway is a powerful therapeutic target in invasive pancreatic cancers, and this current work provides rationale for investigating this line of therapy in the prevention of pancreatic cancers as well.
References:
1. Maitra A, Fukushima N, Takaori K, Hruban RH.
Precursors to invasive pancreatic cancer.
Adv Anat Pathol. 2005 Mar;12(2):81-91. PMID: 15731576
2. Hustinx SR, Leoni LM, Yeo CJ, Brown PN, Goggins M, Kern SE, Hruban RH, Maitra A.
Concordant loss of MTAP and p16/CDKN2A expression in pancreatic intraepithelial neoplasia: evidence of homozygous deletion in a noninvasive precursor lesion.
Mod Pathol. 2005 Jul;18(7):959-63. PMID: 15832197
3. Prasad NB, Biankin AV, Fukushima N, Maitra A, Dhara S, Elkahloun AG, Hruban RH, Goggins M, Leach SD.
Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells.
Cancer Res. 2005 Mar 1;65(5):1619-26. PMID: 15753353

Johns Hopkins Is Number One Again!!!
- July 2005
THE JOHNS HOPKINS HOSPITAL TOPS U.S.NEWS & WORLD REPORT'S "HONOR ROLL" FOR THE 15th YEAR IN A ROW
For the 15th consecutive year, U.S. News & World Report's annual ranking of American hospitals has placed The Johns Hopkins Hospital at the top of the list.
The magazine's recognition is, as always, a tribute to the Hospital, its dedicated nurses and staff, the School of Medicine's faculty physicians and the community physicians whose contributions are significant.
As we endeavor to rebuild, renew, enrich and expand our services and facilities, and to assure our continued commitment to safety and excellence, such independent evaluations as these rankings are of growing value to patients, the public, referring physicians and insurers.
This year's annual guide ranked American medical centers in 17 specialties to identify hospitals that excel in a variety of difficult areas of care by conducting research, pioneering advanced treatments and bringing the best technology and expertise to bear. Just 176 hospitals scored high enough this year to rank in even a single area out of all 6007 U.S. medical centers, and only 16 accumulated enough points to make it to the Honor Roll reserved for medical centers that ranked at or near the top in at least six specialties. Hopkins placed #3 in Cancer, #1 in Gynecology, # 3 in Digestive Disorders. For a complete list of all rankings, go to www.usnews.com or www.hopkinsmedicine.org.

Joseph C. Monastra Foundation
- July 2005
The Joseph C. Monastra Foundation will hold two fundraisers this summer to benefit pancreatic cancer research at Johns Hopkins. On Saturday July 16, 2005, the foundation will hold the "1st Annual JCM Motorcycle Ride" in Valley View, Ohio. The ride begins and ends at the Quaker Steak and Lube on Canal Road. For more information on this fundraiser contact Grace Saunders at: 330-656-0770.
The Joseph C. Monastra Foundation will hold a second fundraiser- the 4th Annual 5K Run/1 Mile Walk, on Saturday August 6, 2005. This event will be held in Hudson, Ohio. For more information contact 216-623-9933, or [email protected].
The proceeds from both events will benefit pancreatic cancer research at Johns Hopkins.
Check out our fundraising page.

Animation
- June 2005
In an effort to keep the users of this website up to date on some exciting new developments in the pancreatic cancer research labs here at Hopkins, we have added a new animation to our research presentation page of this web site. This animation on nanotechnology describes some of the exciting new nanotechnology research in Anirban Maitra’s pancreatic cancer research lab. The animation was developed by Leslie Leonard, M.A., as a part of her Masters thesis for the Art as Applied to Medicine program here at Johns Hopkins. The animation requires Macromedia Flash Player. We thank Leslie for her beautiful art work, and we hope you find this new addition to our web site educational.


New Book on Pancreatic Cancer
- April 2005
Dr. Ralph Hruban, Director of the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins, is one of the editors of a new book on pancreatic cancer. The book "Pancreatic Cancer" has just been published by Jones and Bartlett (shop at amazon). "Pancreatic Cancer" is co-edited by Drs. VonHoff and Evans, and includes chapters written by many of the experts at Johns Hopkins. All aspects of the disease are covered, from embryology, through genetics and treatment. It is hoped that this new book will form the basis for better patient care and that it will spark new research in this field. The book is written for scientists and physicians and may be too technical for the lay public.

New Technology Developed for the Early Detection of Pancreatic Cancer
- April 2005
There are no early warning signs of pancreatic cancer and there are no early detection tests. As a result, most patients are not diagnosed until after the cancer has spread. Just as there is a PSA test for prostate cancer, so too do we urgently need an early detection test for pancreatic cancer. Dr. James Eshleman at Hopkins has recently developed a new technology that can detect rare DNA mutations (alterations in the DNA sequence) even when these mutations are admixed with much larger numbers of normal DNA sequences (Nat Methods. 2004 Oct 21;1(2):141-147 ). The technology, called "LigAmp" detected the most common mutation found in pancreatic cancer (KRAS2 gene mutations) even when a single mutant KRAS2 gene was admixed with 1,000 normal genes. Dr. Eshleman and colleagues have further shown that LigAmp can be used to detect DNA mutations shed from pancreatic cancers in pancreatic juice samples.
To learn more about this exciting advance see www.jhu.edu/~gazette/2004/08nov04/08typos.html
Reference:
Shi C, Eshleman SH, Jones D, Fukushima N, Hua L, Parker AR, Yeo CJ, Hruban RH, Goggins MG, Eshleman JR. LigAmp for sensitive detection of single-nucleotide differences. Nat Methods. 2004 Oct 21;1(2):141-147

New Potential Target for Therapy
- March 2005
Dr. Anirban Maitra and colleagues at Johns Hopkins have identified a genetic change in pancreatic cancers that has potential therapeutic implications. MTAP is a gene on chromosome 9 and novel chemotherapeutic strategies exploiting the selective loss of MTAP function in cancers have been proposed. The MTAP gene is adjacent to the p16 gene and MTAP and p16 are frequently deleted from the DNA of pancreatic cancers. Dr. Maitra has found these deletions of the MTAP and p16 genes in 30% of pancreatic cancers, suggesting that selected patients with pancreatic cancer may benefit from therapies targeting this loss. Studies are now underway in animal models to test this potential new treatment approach.
Reference:
Hustinx SR, Hruban RH, Leoni LM, Iacobuzio-Donahue C, Cameron JL, Yeo CJ, Brown PN, Argani P, Asfaq R, Fukushima N, Goggins M, Kern SE, Maitra A. Homozygous Deletion of the MTAP Gene In Invasive Adenocarcinoma of the Pancreas and in Periampullary Cancer: A Potential New Target for Therapy. Cancer Biol Ther. 2005 Jan 15;4(1): 83-86.

Goldman Family Trust To Fund New Pancreatic Cancer Research Center at Hopkins
- March 2005
Cancer specialists at Johns Hopkins today announced the start of a collaborative research initiative focused on developing novel means of earlier diagnosis and treatment of pancreatic cancer, which kills nearly 31,000 Americans each year and has one of the lowest survival rates for any type of cancer. With $10 million in funding from the Sol Goldman Charitable Trust, a New York-based philanthropy with historic ties to Baltimore, the new initiative will support a team of more than 12 faculty and young researchers.
Click here for the Sol Goldman Center for Pancreatic Cancer Research Website

Do You Have Pancreatic, Stomach? or Colon Cancer?
- February 2005
Researchers at Johns Hopkins are seeking individuals who have the above cancers and may be experiencing weight loss.
Purpose: To evaluate the benefits of fish oil supplementation to maintain weight in individuals with pancreatic, stomach, and colon cancer.
To qualify for the study, you must:
- Be 18 years of age or older
- Be 6 weeks post-operative
- Be on stable dose of most medications
- Can be on concurrent radiation and chemotherapy
Qualified participants will receive study medication, physical exams, laboratory tests, and evaluation of functional status. Parking vouchers will be provided.
For more information, please call the Clinical Trials Unit at 410-502-0033 or email [email protected]
2004 ▼
Our Sincere Congratulations to Dr. Kern!
- December 2004
Our congratulations go out to Dr. Scott Kern. Dr. Kern, a Professor of Oncology and Pathology here at Johns Hopkins, was awarded the first Louis A. Cochet Award for Outstanding Pancreatic Cancer Research. Dr. Kern received this award at a meeting on pancreatic cancer held in Philadelphia on December 1, 2004. This award recognizes Dr. Kern's contribution to the field, including the discovery of the pancreatic cancer gene (DPC4), an advance that led to the discovery of the second breast cancer gene (BRCA2), and for his life's work which has demonstrated that pancreatic cancer is fundamentally a genetic disease (a disease caused by damage to the DNA).

Beta Sigma Donation
- November 2004
The Johns Hopkins Pancreas Cancer Research laboratory of Dr. Christine Iacobuzio-Donahue was awarded a grant of $25,000 at the 2004 National Convention of Sigma Beta Sorority, Inc. which met on October 23, 2004. Sigma Beta is a national philanthropic organization that has donated to various deserving organizations since its founding in 1923. This grant will support Dr. Iacobuzio-Donahue's research that strives to better understand the unique molecular features of pancreatic cancer. Information gathered from her research will form a basis for the development of improved methods to treat this lethal disease.

A Fond Farewell
- September 2004
With a fond farewell, we wish Kieran Brune, the National Familial Pancreas Tumor Registry (NFPTR) Coordinator from June 2000 to September 2004, the best of luck as she begins medical school at the University College of Dublin, Dublin, Ireland. While at Johns Hopkins in her position as NFPTR coordinator, Kieran has seen the registry grow exponentially during her tenure. Over 1400 families are now enrolled in our studies of familial pancreatic cancer. Kieran took the initiative on a number of projects, including the publication of the first newsletter as well as a leadership role in Johns Hopkins collaborations with the national multi-center consortium (PACGENE). She also attended national meetings to represent Dr. Hruban and the NFPTR.
Kieran's compassionate and caring attitude is contagious and touched many of the family's lives in the registry. We wish her continued success in her new endeavors at medical school.
Please join us in welcoming Kieran's replacement, Miriam Tillery. Miriam has spent the past four years working in colon cancer research as a study coordinator. Joining the National Familial Pancreas Tumor Registry as the new coordinator is an exciting opportunity for Miriam to work with patients and their families in their battle against this terrible disease.

Progress on BRCA2/Fanconi gene mutations in pancreatic cancer
- August 2004
Nine years ago, the Kern Laboratory found mutations of a new gene. Soon, this laboratory and others working in other tumor systems found that mutations of the gene were often inherited, raising the risk for pancreatic, ovarian, and breast cancer when an individual inherits one bad copy of the gene. This was the second gene found to cause inherited breast cancer, thus leading to the gene name, BRCA2.
Last year, postdoctoral fellow Michiel van der Heijden of the Kern Laboratory, followed up on that earlier discovery. They knew that BRCA2 was one of the genes that gives rise to a rare syndrome, Fanconi anemia, when two bad copies are inherited by an individual. Fanconi anemia causes skeletal abnormalities and progressive bone marrow failure, and genes other than BRCA2 are responsible for most cases of the syndrome. In each case, one needed to inherit two bad copies in order to develop the syndrome. But what if an individual only inherited one bad copy, a condition that was previously thought to be lacking any association with disease? Was it possible that some of these other Fanconi genes might play a role in pancreatic cancer? Dr. van der Heijden found the answer to be "Yes!" Two of the Fanconi genes, FANCC and FANCG had mutations in a number of pancreatic cancers. Some of these mutations are inherited, meaning that individuals were inheriting a risk for pancreatic cancer. Fanconi anemia gene mutations are found in about 1 in 300 persons in the general population, and in 1 in about 75 Ashkenazi Jews. It remains to be determined how much the risk of cancer increases for such persons, and whether cancers other than pancreatic cancer would be included in the higher risk. Dr. van der Heijden and colleagues had another interesting finding; three of the nine persons whose pancreas cancer had young onset (less than 50 years of age) had such mutations. He perhaps had discovered a fairly common cause of young-onset pancreatic cancer.
At the time, there were no easy tests for the kinds of Fanconi gene mutations now being studied, but such tests may become available in the future. Such testing is likely to be of clinical importance. Cells that are defective in the Fanconi genes are known from other research to be highly sensitive to certain chemicals. In this month's issue of The American Journal of Pathology, Dr. van der Heijden and colleagues report that the Fanconi-deficient pancreatic cancer cells are especially susceptible to the toxic effects of the anticancer drugs mitomycin and cis-platin. If may be possible in the future to recommend individualized therapeutic regimens for patients with these mutations. More research in this exciting new area is underway.

Johns Hopkins Is Number One Again!!!
- July 2004
THE JOHNS HOPKINS HOSPITAL TOPS U.S.NEWS & WORLD REPORT'S "HONOR ROLL" FOR THE 14th YEAR IN A ROW
For the 14th consecutive year, The Johns Hopkins Hospital has topped U.S. News & World Report's rankings of American hospitals.
This year's annual guide reports results of a survey of a hospital's reputation in 17 medical specialties among a national sample of doctors, along with analysis of objective indicators such as death rates, technology, nurse staffing, service mix and discharge planning.
Hopkins again ranked in the top 10 in 16 of the 17 specialty categories listed. In addition to landing at the top of the honor roll, the Hospital ranked #1 in Gynecology, Otolaryngology and Urology; #2 in Geriatrics, Kidney Disease, Neurology/Neurosurgery, Ophthalmology and Rheumatology; #3 in Cancer, Digestive Disorders, Hormonal Disorders, Pediatrics, Psychiatry and Respiratory Disorders; #4 in Heart/Heart Surgery and Orthopedics; and #13 in Rehabilitation.
In a letter announcing the rankings to all employees, Johns Hopkins Medicine Dean/CEO Edward D. Miller, M.D., and Ronald R. Peterson, president of The Johns Hopkins Hospital and Health System, said the recognition is "especially welcome as we labor to build, renew, enrich and expand our services, facilities and safety initiatives." And they again emphasized to all faculty and staff that "neither your contributions nor the magazine's recognition of your commitment is taken for granted."
"While we say it so often, it is still true that this is really a tribute to our Hospital, its wonderful nurses and staff, the School of Medicine's faculty physicians and the many community physicians with whom we have close ties," they added. "It is a worthy acknowledgment of the innovative and compassionate patient care that is Hopkins's hallmark -- and of the people who make that kind of care possible."
Miller and Peterson also commented that "such independent evaluations as these rankings are of growing value to patients, the public, referring physicians and insurers."
Finally, they noted once again that "our presence on the list puts us in great company, and we congratulate all of the medical centers on the magazine's honor roll."
Coming in at #2 on the honor roll was the Mayo Clinic. Rounding out the Honor Roll list, in rank order, are Massachusetts General Hospital; Cleveland Clinic; UCLA Medical Center; Duke University Medical Center and University of California San Francisco (tie); Barnes-Jewish Hospital, St. Louis; New York-Presbyterian Hospital and University of Washington Medical Center, Seattle (tie); University of Michigan Medical Center, Ann Arbor; Brigham and Women's Hospital, Boston; Hospital of the University of Pennsylvania, Philadelphia; and Stanford Hospital and Clinics.

Screening for Pancreatic Neoplasia in High-risk Individuals: An EUS-based Approach
- July 2004
In the July issue of Clinical Gastroenterology Hepatology, Marcia Canto and colleagues from Johns Hopkins report the results of screening for pancreatic cancer in asymptomatic (patients without symptoms) individuals known to be at increased risk because of their family history. Endoscopic ultrasound (EUS) was used. In this technique a small tube with an ultrasound device at the end is inserted through the patient's nose and into the patient's stomach and duodenum. The ultrasound device can then be used to image the pancreas. Thirty-eight patients were screened in Dr. Canto's study and six pancreatic masses were found. Several of these patients went to surgery and one was found to have an early cancer and another a precancerous tumor (intraductal papillary mucinous neoplasm). Therefore, 5.3% or 1 in 20 patients was found to have a clinically important pancreatic mass. This study therefore represents the first step towards demonstrating that screening for early pancreatic cancer is possible.
Clin Gastroenterol Hepatol. 2004 Jul; 2(7):606-21

National Familial Pancreatic Tumor Registry Update
- April 2004
Dr. Klein and colleagues (Cancer Research 64:2634-2638), followed 838 families who participated in the National Familial Pancreatic Tumor Registry to determine how likely were new pancreatic cancer to develop in these families. 22 new pancreatic cancer cases developed in these families, after the family had entered the registry. Dr. Klein found that individuals with three or more first-degree relatives with pancreatic cancer (brothers, sisters, parents or children) were at a 32-fold increase risk of developing pancreatic cancer. Individuals with two first-degree relatives with pancreatic cancer had a 6.5-fold increased risk. This increase in risk was even higher among family members who smoked cigarettes. The results of this study further establish that pancreatic cancer does cluster in families. Additionally, these results help to identify individuals who may benefit from screening for the early signs of pancreatic cancer once reliable screening tests are developed.
To learn more about how you can join the National Familial Pancreas Tumor Registry contact the registry coordinator Kieran Brune (410-955-3502 or e-mail [email protected]).

Recent publications from Dr. Iacobuzio-Donahue
- April 2004
Dr. Iacobuzio-Donahue recently published two exciting articles in Cancer Research. The first article, published in the December 15 issue (Cancer Research 2003; 63:8614-22) describes the largest, most comprehensive study to date of gene expression (the genes made) in pancreatic cancer. Close to 100 samples were analyzed using the most current gene chip (Affemetrix U133). Dr. Iacobuzio-Donahue and colleagues discovered 142 potential new markers of pancreatic cancer. Already, Dr. Koopmann has shown that one of these markers, called "osteopontin," is elevated in the blood of patients with pancreatic cancer (Cancer Epi Biomarkers Prev, 2004; 13:487-91) and Dr. Nichols has demonstrated that another of these markers, claudin 4, may be a useful therapeutic target (Am J Clin Pathol. 2004; 121:226-30).
In the second paper published by Dr. Iacobuzio-Donahue (Cancer Research 2004; 64:871-875) she describes the largest genetic analysis conducted to date of the DNA changes in pancreatic cancer. Dr. Iacobuzio-Donahue and colleagues conducted a large-scale "allelotypes" (an analysis of DNA losses in a cancer) on a series of pancreatic cancers and they discovered a number of hot spots of DNA alterations in pancreatic cancer. These hot spots will help other scientists identify the genes that are targeted for inactivation in pancreatic cancer. An understanding of these genes, in turn, may lead to a better understanding of why pancreatic cancer develops and how to treat it.
References
Iacobuzio-Donahue CA, van der Heijden MS, Baumgartner MR, Troup WJ, Romm JM, Doheny K, Pugh E, Yeo CJ, Goggins MG, Hruban RH, Kern SE. Large-scale allelotype of pancreaticobiliary carcinoma provides quantitiative estimates of genome-wide allelic loss. Cancer Res. 64: 871-875, 2004.
Koopmann J, Fedarko NS, Jain A, Maitra A, Iacobuzio-Donahue C, Rahman A, Hruban RH, Yeo CJ, Goggins M. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 13:487-91, 2004.
Iacobuzio-Donahue CA, Cong J, Parmiagiani G, Yeo CJ, Hruban RH, Kern SE. Missense mutations of MADH4: Characterization of the mutational hot spot and functional consequences in human tumors. Clin Cancer Res. 10:1597-1604; 2004.
Nichols LS, Ashfaq R, Iacobuzio-Donahue CA. Claudin 4 protein expression in primary and metastatic pancreatic cancer: support for use as a therapeutic target.Am J Clin Pathol. 121(2):226-30, 2004.
2003 ▼
Fundraisers to Benefit Hopkins Pancreas Cancer Research
- November 2003
Two fundraising events were held in different areas of the country to benefit pancreatic cancer research at John Hopkins. These fundraisers honored two very special men, helped to raise critical money to fight this dreadful disease, and celebrated November as Pancreas Cancer Awareness Month.
"The Second Annual Ride to Make a Difference for Pancreatic Cancer" was held in Atlanta, Georgia by the Joseph C. Monastra Foundation for Pancreatic Cancer Research. Held on a beautiful day with a full police escort of 5 motorcycle cops, the 75-mile ride was safe, smooth and enjoyable. The generosity shown by the biker community of Georgia was overwhelming with one auction item being donated back after being sold only to be sold again. To learn more about this remarkable family and their fundraising efforts, visit our Web site's overview of the Monastra family and Foundation.
The George Rubis Endowment for Pancreatic Cancer Research held "The First Annual Run for George" in Englewood Cliffs, New Jersey. The 5K race, silent auction, food and carnival games were enjoyed by the family, friends, and the local community in honor of George Rubis' memory and to support pancreatic cancer research at Johns Hopkins. Kieran Brune, Coordinator of the National Familial Pancreas Tumor Registry, ran the 5K event and spoke to those in attendance.

New Pathway for Cancer Discovered in Hedgehog
- September 2003
Friends — I wanted to update you on a remarkable discovery from the pancreatic cancer research team here at Johns Hopkins. Today the journal Nature released an on-line version of a paper by Drs. Berman, Maitra and Beachy in which they demonstrate the activation of the "sonic hedgehog gene" in pancreatic and biliary cancers (https://www.nature.com/nature). The complete print version will appear in a few weeks in Nature.
"Sonic hedgehog" is part of a cell pathway that plays an important role in embryogenesis. Cell pathways are groups of proteins within cells that interact/communicate with each other. Embryogenesis is the formation of the embryo. Dr. Beachy discovered several of the key components of this pathway, studying, of all things, fruit fly genetics. It was shown that alterations in the sonic hedgehog pathway caused fruit fly larva to look like, you guessed it, the computer game character "sonic hedgehog". Dr. Beachy's team then went on to show that this same pathway is important in human embryogenesis and that the sonic hedgehog pathway plays a key role in maintaining stem cells in our bodies. Dr. Beachy was elected to the National Academy of Science for this work. Now, Drs. Beachy, Maitra and Berman team together to show that the sonic hedgehog pathway is activated in human cancers, including cancers of the pancreas and biliary tree. Furthermore, they show that when they block the pathway using a drug called cyclopamine, that they completely block tumor growth.
This paper is very exciting to me for several reasons. First, it helps us understand the fundamental biology of pancreatic and biliary cancer. Their data show that a pathway that regulates stem cells is altered in these cancers (for scientists this is a very exciting idea). Second, the discovery of this pathway's role in human pancreatic and biliary cancer opens an entire new area for treating these cancers-- targeting pancreatic and biliary cancers using drugs, such as cyclopamine, that specifically inhibit the sonic hedgehog pathway. The team now plans to test a large panel of blockers ("inhibitors") of the sonic hedgehog pathway to determine which inhibitor provides the maximum anti-tumor effect and the minimum side effects. Third, the study is an example of what, in my opinion, makes Hopkins such a special place- the willingness of creative scientists from very diverse fields to work together to tackle big problems. Fourth, the creation of the cell lines and cancer xenografts that were central to this research was made possible by private donations. This is a wonderful example of how private philanthropy can support cutting edge research and have a significant impact in the war on cancer.
Ralph H. Hruban, M.D.
Professor of Pathology and of Oncology

Johns Hopkins Is Number One Again
- July 2003
THE JOHNS HOPKINS HOSPITAL TOPS U.S.NEWS & WORLD REPORT'S "HONOR ROLL" FOR THE 13th YEAR IN A ROW
For the 13th consecutive year, Johns Hopkins comes in at the very top of the list in the U.S. News & World Report's annual ranking of American hospitals. Hopkins ranked in the top 10 in 16 of 17 specialties, including cancer. Ratings are based on reputation (ranking by randomly selected physicians), mortality rates, and hospital factors such as discharges, ratio of nurses to beds, technology services, hospice/palliative care services, and whether or not the hospital is an NCI Cancer Center.

New Targets for Aberrant Methylation in Pancreas Cancer
- July 2003
Research by Norihiro Sato MD, PhD in the laboratory of Dr. Michael Goggins has led to the discovery of multiple genes that undergo silencing by methylation in pancreatic cancer. Knowledge of these genes provides us with a better understanding of the role of DNA methylation in pancreatic cancer development. The authors also showed that these abnormally methylated genes can be detected in pancreatic juice from patients with pancreatic cancer and raising hopes that their detection could aid in the early diagnosis of pancreatic cancer.
Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays.
Cancer Res. 2003 Jul 1;63(13):3735-42.

Anti-Cancer Drug Can Lead to Cancer Invasion
- July 2003
In the February 19th issue of the Journal of the National Cancer Institute, Sato and colleagues report an unexpected adverse effect of an anti-cancer drug that is used to remove "methyl" groups (carbon-hydrogen) from DNA. The authors find that this drug can switch on certain genes (matrix metalloproteinases) that lead to cancer invasion. This effect is the result of the removal of methyl groups that function to keep these genes switched off in pancreas cells.
Sato N, Maehara N, Su GH, Goggins M.
Effects of 5-aza-2'-deoxycytidine on matrix metalloproteinase expression and pancreatic cancer cell invasiveness.
J Natl Cancer Inst. 2003 Feb 19;95(4):327-30.

Pancreatic Cancer Linked to Errant Reactivation of Embryo Cell Pathway
- June 2003
Research by Johns Hopkins Kimmel Cancer Center specialists has uncovered a novel pathway in the origin of pancreatic cancers. The Notch pathway, normally turned off in adults, can be turned on after injury to the pancreas.
The findings of Dr. Steven Leach et al. are reported in the June 23, 2003, issue of Cancer Cell.

Washington D.C. PanCAN Visits Johns Hopkins
- June 2003
On Saturday June 7, 2003 close to 25 members of the Washington D.C. PanCAN group visited Johns Hopkins. The visit was organized by Mary Zapor from PanCAN and Sandy Markowitz from Hopkins. The visitors toured some of the labs, spoke with the scientists, and listened to talks on the clinical treatment of pancreatic cancer from Drs. Yeo and Laheru. In the end, the group had an informal lunch with the doctors and scientists. The morning was educational, but most importantly it motivated the Hopkins docs to work that much harder in the battle against pancreatic cancer, and it gave the PanCAN visitors a sense of the breadth and depth of research going on at Hopkins and a sense of excitement that exists now in the research labs at Hopkins.
The visit demonstrates the value of patient advocates, physicians and scientists working together.
If you would like to learn more about how you can support pancreatic cancer research at Johns Hopkins visit our Support Page.


Animation Explaining Mouse Models of Pancreatic Cancer
- June 2003
A number of you have asked us to explain the important, but very complex, work that Gloria Su, PhD does in her lab developing mouse models of pancreatic cancer. One of the Art as Applied to Medicine students here at Johns Hopkins, Christian Rose, has developed a web animation to explain Dr. Su's research. We have posted it on the research presentation page of this web site. It is a big file, so you will have to be patient downloading it. We hope you find this animation interesting and educational.

Fanconi Gene Abnormalities in Pancreatic Cancer
- May 2003
Eight years ago while studying pancreatic cancers, the Kern Laboratory found mutations of a new gene. Soon, this laboratory and others working in other tumor systems found that mutations of the gene were often inherited, raising the risk for pancreatic, ovarian, and breast cancer when an individual inherits one bad copy of the gene. This was the second gene found to cause inherited breast cancer, thus leading to the gene name, BRCA2.
In a paper published in the May 15, 2003 issue of the journal Cancer Research, Dr. Michiel van der Heijden and colleagues, also working in the Kern Laboratory, is following up on that earlier discovery. They knew that BRCA2 was one of the genes that gives rise to a rare syndrome, Fanconi anemia, when two bad copies are inherited by an individual. Fanconi anemia causes skeletal abnormalities and progressive bone marrow failure, and genes other than BRCA2 are responsible for most cases of the syndrome. In each case, one needed to inherit two bad copies in order to develop the syndrome. But what if an individual only inherited one bad copy, a condition that was previously thought to be lacking any association with disease? Was it possible that some of these other Fanconi genes might play a role in pancreatic cancer? Dr. van der Heijden found the answer to be "Yes!"
The scientists studied two of the Fanconi genes, FANCC and FANCG. Inherited and new mutations were found in a number of pancreatic cancers. Some of these mutations are inherited, meaning that individuals were inheriting a risk for pancreatic cancer. Fanconi anemia gene mutations are found in about 1 in 300 persons in the general population, and in 1 in about 75 Ashkenazi Jews. It remains to be determined how much the risk of cancer increases for such persons, and whether cancers other than pancreatic cancer would be included in the higher risk. Dr. van der Heijden and colleagues had another interesting finding; three of the nine persons whose pancreas cancer had young onset (less than 50 years of age) had such mutations. He perhaps had discovered a fairly common cause of young-onset pancreatic cancer.
There are no easy tests for the kinds of Fanconi gene mutations now being studied, but such tests may become available in the future. Such testing is likely to be of clinical importance. Cells that are defective in the Fanconi genes are known from other research to be highly sensitive to certain chemicals. If may be possible in the future to recommend a different therapeutic regimen for patients with these mutations. More research in this exciting new area is needed.

Activin Abnormalities in Pancreatic Cancer
- February 2003
Two years ago while studying pancreatic cancers in the Kern Laboratory, Dr. Gloria Su and colleagues identified mutations in a gene that allows cells to respond to the presence of the extracellular protein, activin. Activin is part of a major system that vertebrate cells use to control populations of cells during development. It now appeared that the adult tissues also used activin for a similar purpose. The cancer cells had lost this form of control by activin by evolving dysfunctional mutations in the type IB receptor for activin that would normally be present on the surface of cells.
In a paper to be published in the March 1, 2003 issue of the journal Cancer Research, Dr. Paula Hempen and colleagues, also working in the Kern Laboratory, have followed up on that earlier discovery. They find additional mutations of another form of the activin receptor, extending the numbers of tumors known to have abnormalities in the activin system. The newly discovered mutations are in the ACVR2 gene, the activin type 2 receptor. The ACVR2 gene mutations were found in nearly all gastrointestinal tumors that had defects in a DNA-repair pathway involved in familial forms of cancer, including families at high risk of colorectal, pancreatic, and endometrial cancers.
These activin receptor mutations seem to be a fundamental change, without which the tumors perhaps could not occur. Dr. Byungwoo Ryu and colleagues in the Kern Laboratory therefore studied the genes that respond to activin signals to uncover the ways in which the cells are regulated by activin. Using a high-density gene expression screen, they studied gene expression changes characteristic of activin. Some of the genes regulated include genes that directly control cell division. This work is currently in press at the journal Cancer Biology & Therapy, and represents the largest study of gene responses to activin published to date.

New Animation Details Anti-PC Vaccine
- January 2003
We've added an animation to our Website to describe the pancreas cancer vaccine being developed by Dr. Liz Jaffee here at Johns Hopkins. Created by a Medical Arts student, Helen MacFarlane, the animation helps the layperson understand how the vaccine is made and how it works. The vaccine is currently in a Phase 2 trial and is still enrolling participants. Click here to view the animation and learn more about this vaccine.
2002 ▼
Over 1 Million Dollars Donated!
- December 2002
We are pleased to announce that to date over 1 million dollars has been donated to pancreatic cancer research at Johns Hopkins by the users of this Web site! This is a truly remarkable achievement and it has had a significant impact on the war against pancreatic cancer. Since 1995 over 4700 donors have contributed! Some have given as little as $5 and others over $250,000. Almost all were given to honor a friend or loved one who either is battling pancreatic cancer or who lost the battle against this disease.
As you can see, these donations have added up and the 1 million dollars raised has allowed us to hire three talented scientists and provide equipment and space necessary for their research. Michael Goggins M.D. is a gastroenterologist and he has established a laboratory dedicated to developing an early detection test for pancreatic cancer. Just as there is a PSA test for prostate cancer, so too do we need a blood test for early pancreatic cancer. Gloria Su, Ph.D. trained in Scott Kern's laboratory and has started her own lab dedicated to developing a mouse model of pancreatic cancer that directly mirrors human disease. This mouse model will greatly facilitate the testing of new treatments and screening tests for pancreatic cancer. Finally Christine Iacobuzio-Donahue M.D., Ph.D. has just joined our faculty and her laboratory will be dedicated to identifying new targets for the treatment of pancreatic cancer. She will do this by applying cutting-edge gene chip technology to a series of well-characterized pancreatic cancers resected here at Johns Hopkins. These three exciting new laboratories exist because of the support of the users of this Web site and, importantly, all three of these scientists have obtained additional grant support for their pancreatic cancer research from private foundations and from the federal government. Thus, your donations have not only allowed new talented young scientists to join the battle against pancreatic cancer, but your donations have also given us a foundation on which to obtain additional support for pancreatic cancer research from other agencies.
We congratulate all of you for your generous support. It is sincerely appreciated and it has made a wonderful difference!

December 2002 Issue of NFPTR News Available On-line
- December 12, 2002
Enrollment in The National Familial Pancreas Tumor Registry (NFPTR) continues to grow. Over 1,000 families now participate in this research registry. A number of you have asked for periodic updates on the research being conducted through the NFPTR. For those of you interested in our studies of why pancreatic cancer runs in some families, the December 2002 issue of NFPTR News is now available on-line. The url for the pdf version of NFPTR News is: 2002 Newsletter.
If you have a strong family history of pancreatic cancer (at least two family members with pancreatic cancer), but have not enrolled in the NFPTR, please consider participating in our research. If you would like to join our research please contact the coordinator of the NFPTR Kieran Brune (410-955-3502 or [email protected]).

Critical Early Step in the Development of Pancreatic Cancer Identified
- November 5, 2002
In the November 2002 issue of the American Journal of Pathology Dr. Tjarda van Heek and colleagues from Johns Hopkins report that the ends of chromosomes (called "telomeres") are dramatically shortened in the lesions that are the precursors to invasive pancreatic cancer (called "PanINs"). Telomeres normally function to keep the ends of chromosomes from "sticking together". When telomeres are too short, chromosome ends stick together and can be damaged when a cell tries to divide. This chromosome damage, in turn, may promote the development of an invasive pancreatic cancer.
The study by van Heek and colleagues suggests that telomere shortening may be a critical early event in the development of a pancreatic cancer and the findings open a whole new area of pancreatic cancer research- the role of telomeres and the mechanisms of telomere shortening in pancreatic cancer.
Reference:
Telomere Shortening Is Nearly Universal in Pancreatic Intraepithelial Neoplasia N. Tjarda van Heek, Alan K. Meeker, Scott E. Kern, Charles J. Yeo, Keith D. Lillemoe,| John L. Cameron, G. Johan A. Offerhaus, Jessica L. Hicks, Robb E. Wilentz, Michael G. Goggins, Angelo M. De Marzo, Ralph H. Hruban and Anirban Maitra American Journal of Pathology. 2002;161:1541-1547

Another Monastra Foundation Fundraiser to Benefit Hopkins Pancreas Cancer Research
- November 2002
Joseph C. Monastra passed away on April 4, 2002 only four short weeks after being diagnosed with Pancreatic Cancer. Joseph dedicated his life and career to his family, friends and to the United States Defense Industry. In honor of Joseph's memory, his family has established the "Joseph C. Monastra Fund for Pancreatic Cancer Research" at Johns Hopkins. The Monastra Foundation held its second fundraiser, "The First Annual Ride to Make a Difference for Pancreatic Cancer", a Motocycle Ride, in Atlanta, Georgia on November 2, 2002. To learn more about this remarkable family and their fundraising efforts, click here.

Aspirin Use May Reduce Risk of Pancreatic Cancer
- August 2002
In the August 7, 2002 issue of the Journal of the National Cancer Institute (volume 94, pages 1168-1171) K.E. Anderson and colleagues from the University of Minnesota report that aspirin might be chemopreventive for pancreatic cancer. They followed over 28,000 post-menopausal women in Iowa from 1992 and 1999. Eighty of these women developed pancreatic cancer. When the investigators looked at self-reported aspirin use among all 28,000 women, they found a trend of decreasing risk of pancreatic cancer with increasing frequency of aspirin use per week. While these data suggest that aspirin use may reduce one's risk of developing pancreatic cancer, you are advised to consult with your personal physician before beginning any treatment course.

Grass-Roots Fundraiser to Benefit Pancreas Cancer Research at Hopkins
- August 2002
The Third Annual Michael Rolfe Research Foundation Gala to benefit pancreatic cancer research at Johns Hopkins will be held on Sunday August 25, 2002 at RAVINIA FESTIVAL, in HIGHLAND PARK Illinois. Cocktails & gathering time will start at 4:30pm, followed by a Gala Dinner in the tent at 5:00 pm and a live concert at 7:00 pm. The concert "Happy Birthday, Doc!" will feature Doc Severinsen and the Ravinia Festival Orchestra with Erich Kunzel Conducting. Limited tickets available.
For more information please contact: Michael Rolfe Research Foundation, P.O. Box 1762, Highland Park, IL 60035 847-432-4926. More details

Johns Hopkins Hospital Rated #1 Again
- July 2002
THE JOHNS HOPKINS HOSPITAL TOPS U.S.NEWS & WORLD REPORT'S "HONOR ROLL" FOR THE 12th YEAR IN A ROW
For the 12th consecutive year, Johns Hopkins comes in at the very top of the list in the U.S. News & World Report's annual ranking of American hospitals. Hopkins was also rated as exceptional in 16 of 17 specialties, including cancer. Ratings are based on reputation (ranking by randomly selected physicians), mortality rates, and hospital factors such as discharges, ratio of nurses to beds, technology services, hospice/palliative care services, and whether or not the hospital is an NCI Cancer Center.
The methodology section of the report refers to a journal article featured in our What's New section a few months ago [What's New April 22, 2002] regarding the role of patient volume in hospital excellence. Specifically, the report points out that patients undergoing pancreatic cancer surgeries do better in hospitals with large volumes.


Pancreatic Cancer Fundraiser to Honor Football Player's Father
- June 2002
The Jeffrey M. Zgonina Foundation will hold its first annual fundraiser on June 30, 2002. The fundraiser will be held at the Stonegate Banquet and Conference Center in Hoffman Estates, Illinois. Jeffrey Zgonina is a football player on the St. Louis Rams and this fundraiser will honor his father, Kaz Zgonina, who died from pancreatic cancer in September 2000. The fundraiser will begin at 4:00 p.m. with cocktails, hors d'oeuvres and a silent auction, followed by dinner, entertainment and a live auction. Auction items include football memorabilia collected by Jeffrey Zgonina. The proceeds from this event will benefit the Pancreatic Research Program at Johns Hopkins University. In particular, the monies raised at the Jeffrey Zgonina Foundation Fundraiser will help a young physician-scientist at Johns Hopkins, Dr. Christine Iacobuzio-Donahue, start a brand new pancreatic cancer research lab at Johns Hopkins.

Patients Undergoing Whipple Surgery Can Significantly Reduce Their Risk of Operative Death by Selecting a High-Volume Center
- April 2002
In the April 11, 2002 issue of the New England Journal of Medicine Dr. Birkmeyer and colleagues from Department of Veterans Affairs, Vermont, report their analysis of surgical mortality in the United States (N Engl J Med 2002 Apr 11;346(15):1128-37). Using information from the national Medicare claims database and the Nationwide Inpatient Sample they examined the relationship between hospital volume (total number of procedures performed per year) and mortality (death in hospital or within 30 days) for a variety of surgical procedures. The mortality rate for Whipple procedures (pancreaticoduodenectomy) at low-volume centers (16.3%) was much greater than the mortality rate at high-volume centers (3.8%). From their analyses the authors conclude that patients "can significantly reduce their risk of operative death by selecting a high-volume hospital." High-volume centers were defined in this study as centers that perform more than 16 whipples per year. Last year close to 240 whipples were performed at Johns Hopkins.
Reference:
Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med 2002 Apr 11;346(15):1128-37.

New Markers of Pancreatic Cancer Discovered Using Global Gene Expression Technology
- April 2002
In the April issue of the American Journal of Pathology (Am J Pathol 2002 Apr;160(4):1239-49) Dr. Iacobuzio-Donahue and colleagues from Johns Hopkins report the discovery of 69 genes that are made at higher levels in pancreatic cancers than in normal tissues. Dr. Iacobuzio-Donahue used recently "Gene Chips" to compare the genes expressed (made) in a large series of pancreatic cancers to the genes expressed (made) in normal tissues. When she did this she identified 97 genes that were highly overexpressed (made at very high levels in cancers compared to the normal samples). 28 of these genes had been reported before, leaving a remarkable 69 new genes selectively overexpressed in pancreatic cancer. These 69 new genes have immediate potential as novel therapeutic targets and tumor markers of pancreatic cancer. The group at Hopkins is now systematically evaluating each these 69 new genes.
Reference:
Am J Pathol 2002 Apr;160(4):1239-49

Rob Larsen Bike Ride to Support Pancreatic Cancer Research at Hopkins
- March 2002
Rob Larsen will ride his bicycle 100 miles on June 15, 2002 in the hope that it will inspire donors to make a contribution toward pancreatic cancer research at Johns Hopkins University. Rob is making this ride in honor of his mother, Suzanne Larsen, who died from the disease in 1999. Rob is calling this fund raiser "The Ride For Mom 1" because he hopes to make it the first of many annual rides dedicated to raising money for pancreatic cancer research at Hopkins.

Hopkins Researchers Discover New Marker for Survival
- March 2002
In the December issue of Clinical Cancer Research, Dr. Tascilar and colleagues report the discovery of an important marker of survival among a large number of patients who undergo surgical resection of their pancreatic cancer at Johns Hopkins Hospital. Working in the laboratory of Dr. Goggins, Dr. Tascilar found that patients whose pancreatic cancers still had a normal SMAD4 (DPC4) gene and protein lived significantly longer than those patients whose pancreatic cancers had already lost SMAD4 at the time of surgery. These findings highlight the important role of SMAD4 in preventing cancer growth and highlight the need for treatments that can function like SMAD4 in cancers that have lost the function of this gene. The presence or absence of normal SMAD4 in a cancer can be determined by routine immunohistochemistry and its measurement can provide prognostic information for patients.

Hopkins Researchers Discover Genes Silenced in Pancreatic Cancer Development
- March 2002
In the December issue of Cancer Research, Dr. Ueki and colleagues report the discovery of a number of genes that become silenced during pancreatic cancer development by DNA methylation. Methylation refers to the addition of an extra carbon atom to DNA, and methylation of a gene can switch off its function. Methylation, like mutation, frequently occurs in cancer and is a common way in which cancer-preventing (tumor suppressor) genes are inactivated in cancers. Working in Dr. Goggins' lab and using a technique known as RDA (representational difference analysis), Dr Ueki compared methylation patterns in normal pancreas with those in pancreatic cancers, and found that the gene ppENK (also called "enkephalin") is silenced by methylation in ~90% of pancreatic cancers. Enkephalin suppresses the growth of pancreas cancer cells and switching off enkephalin by methylation increases cancer growth. Importantly, since it is possible to detect abnormal methylation of DNA, it is hoped that diagnostic tests can be developed to detect methylated encephalin in pancreatic juice, thereby permitting the early detection of pancreatic cancer. Finally, the authors of this study are much indebted to PANCAN (the PANcreatic Cancer Action Network) and to the friends and family of Michael Rolfe who provided generous donations to make this research possible.

Advance Made in Hunt for Familial Pancreas Cancer Gene
- March 2002
In this month's issue of the American Journal of Human Genetics, Drs. Teresa Brentnall (Fred Hutchinson Cancer Research Center), David Whitcomb (University of Pittsburgh School of Medicine) and colleagues, report that they have narrowed done the search for the gene responsible for the aggregation of pancreatic cancer in a family (called "Family= X"). Using a technique called "linkage analysis" Drs. Brentnall and Whitcomb were able to show that the gene in this family most likely resides on chromosome 4 at a chromosome location called "4q32-34". Their finding is exciting because, if the gene can be found and if the gene in this family is the same as the gene responsible for the aggregation of pancreatic cancer in other families, then it will help us understand why pancreatic cancer runs in some families and, importantly, it will help us predict who is at increased risk for developing pancreatic cancer and who is not. If you are from a family in which more than one family member has been diagnosed with pancreatic cancer and you would like to help scientists at Johns Hopkins with their research you may wish to consider participating in the National Familial Pancreas Tumor Registry (NFPTR) at Johns Hopkins. Please contact Kieran Brune, Coordinator, at 410-955-3502 or [email protected]. Your participation in this research may help us discover the "familial pancreas cancer gene".

New Marker of Pancreatic Adenocarcinoma Discovered by Serial Analysis of Gene Expression (SAGE)
- January 2002
In the December issue of Clinical Cancer Research, Dr. Argani and colleagues from Johns Hopkins describe the discovery of a new marker of pancreatic adenocarcinoma. Dr. Argani and colleagues used the same technique developed at Johns Hopkins, serial analysis of gene expression (SAGE), as was used to discover another new marker of pancreatic cancer, prostate stem cell antigen (PSCA) (See What's New, June 7, 2001). Again, they used an online database of SAGE data on a variety of tissues and cancers (https://www.ncbi.nlm.nih.gov/SA... ) to identify genes that are turned on in pancreatic cancer. The gene identified, mesothelin, has been shown to be expressed in cancers of the lung, cervix, and ovary, but had not been analyzed in pancreatic cancer. Interestingly, it appears that this protein was actually initially described in a pancreas cancer cell line seven years previously, but this information had been ignored until the current study. Using techniques to study RNA expression (reverse transcriptase PCR and in situ hybridization) and protein expression (immunohistochemistry), Dr. Argani and colleagues found mesothelin to be overexpressed in over 90% of pancreatic adenocarcinomas with minimal to no expression in normal tissues.
These findings are exciting for several reasons. First, they confirm the ability of SAGE to identify new tumor markers. Second, mesothelin, because it is selectively overexpressed in pancreatic cancers, may be useful as a new marker for pancreatic cancer. Third, mesothelin has been extensively studied and is a potential target for immune treatment of ovarian cancer. The demonstration of mesothelin overexpression in pancreatic cancer suggests that a similar approach could be used. Since pancreatic cancer cells overexpress mesothelin, immunotherapy against mesothelin could be helpful to treat pancreatic cancer.
Finally, the authors of this study are much indebted to the friends and family of Michael Rolfe. This work was supported in large part by generous donations from the family and friends of Michael Rolfe, demonstrating again the power of private giving to advance cancer research.
Reference:
Clin Cancer Res. 2001 Dec;7(12):3862-8

New Study Highlights the Importance of the Body's Reaction to Pancreatic Cancer
- January 2002
In this month's issue of the American Journal of Pathology, Dr. Iacobuzio-Donahue and colleagues from Johns Hopkins report an extensive study of the body's reaction to pancreatic cancer (American Journal of Pathology 2002, Vol. 160, pages 91-99; https://pubmed.ncbi.nlm.nih.gov/11786403/). Dr. Iacobuzio-Donahue and colleagues in the laboratory of Dr. Scott Kern used the recently developed technique of "serial analysis of gene expression" (SAGE) to identify nearly a hundred genes that are overexpressed (made at high levels or "turned on") in pancreatic carcinoma. Dr. Iacobuzio-Donahue didn't stop there. She selected twelve of these genes and asked, exactly where, in what cells, are these genes turned on in resected pancreatic cancer? In so doing, she was able to develop a remarkable picture of the "architecture" of gene expression in pancreatic cancer. Eight of the genes she identified were made within the small blood vessels and scar tissue (an exuberant response of connective tissues of the patient, termed the "desmoplastic" response) that become intermixed with the cancer. It is an intriguing fact about pancreatic cancer that this scarring response makes up most of the volume, or space, occupied by the tumor - the ability to understand it better would be of considerable help in understanding how these cancers grow and destroy neighboring areas. The invasive cancer cells, themselves, specifically made four additional genes. This set the stage for an unanticipated and eye-opening finding: the patterns of gene expression that Dr. Icaobuzio-Donahue identified in these cancers suggested an important communication between the cancer cells and the adjacent scar tissue and blood vessels. In one example, a gene called connective tissue growth factor was expressed by the cancer cells and may explain why the scar tissue (also called connective tissue) is so prominent in pancreatic cancer.
Importantly, not only does Dr. Iacobuzio-Donahue provide insight into the basic nature of the body's reaction to a pancreatic cancer, but it also suggests a number of new targets for the development of future therapeutics to treat this disease.
Reference:
Iacobuzio-Donahue CA, Ryu B, Hruban RH, Kern SE. Exploring the host desmoplastic response to pancreatic carcinoma : gene expression of stromal and neoplastic cells at the site of primary invasion. Am J Pathol. 2002 Jan;160(1):91-9.

Precursor to Invasive Colloid Cancers of the Pancreas Identified-
- January 2002
There are several variants of ductal adenocarcinoma of the pancreas. One of those variants is called colloid or mucinous carcinoma. This entity is defined by adenocarcinomas comprised largely of malignant cells floating free within pools of mucin. While this type of adenocarcinoma has already been well studied in organs like breast, colon, and prostate, it has not been well studied in the pancreas, duodenum, or ampulla of Vater. In the most recent issue of the American Journal of Surgical Pathology, Wilentz, et al. try to fix this problem by analyzing a set of 39 colloid carcinomas that arise in the pancreas, duodenum, and ampulla of Vater. They find that the overwhelming majority of these carcinomas (38 of 39, 97%) arise from a low-grade precursor, either an intraductal papillary mucinous neoplasm (in the pancreas) or a tubulovillous adenoma (duodenum or ampulla). In addition, they find that the colloid differentiation is not as important a prognostic factor for patients with pancreatic cancer as are other factors (for example, tumor location, tumor stage or margin status after resection). Nevertheless, because colloid carcinomas almost always arise within a low-grade component, the findings in this paper are significant. If the low-grade component is discovered before cancer develops, a patient will have a much better chance of survival. We at Johns Hopkins are now working on ways to detect these low-grade components at early stages.
2001 ▼
Unraveling the genetic changes in pancreatoblastomas
- November 2001
While scientists have made great strides in advancing our understanding of pancreatic ductal adenocarcinoma, little is known about rarer tumors that arise in the pancreas. In this month's issue of the American Journal of Pathology, S. Abraham and colleagues from Johns Hopkins report a detailed molecular genetic analysis of pancreatoblastomas. Pancreatoblastoma is a rare pancreatic tumor with a distinctive microscopic appearance that generally affects infants and young children (see the FAQ section of this Web site for more information on pancreatoblastomas and other rarer variants of pancreas cancer). Dr. Abraham analyzed a series of nine pancreatoblastomas for genetic alterations (changes in the DNA sequence of the tumors). She found three interesting things. First, pancreatoblastomas are genetically very different from the more common ductal adenocarcinomas of the pancreas. Most ductal adenocarcinomas harbor mutations in the k-ras, p53 and DPC4 genes, while pancreatoblastomas do not. Instead, pancreatoblastomas show alterations (mutations) in the beta-catenin/APC genes. 2) Dr. Abraham also showed that chromosome 11p is frequently altered in pancreatoblastomas. Chromosome 11p is frequently altered in hepatoblastomas (a rare pediatric tumor in the liver), suggesting that pancreatoblastomas are more closely related to hepatoblastomas than they are to pancreatic ductal adenocarcinomas. 3) Finally, one of the patients included in Dr. Abraham's series of pancreatoblastomas had the clinical syndrome called "familial adenomatous polyposis" or "FAP". Patients with familial adenomatous polyposis develop numerous polyps in their colon at an early age and Dr. Abraham demonstrates that they can also develop pancreatoblastomas.
Dr. Abraham's study is an important advance in our understanding of some of the rarer variants of pancreas cancer. Dr. Abraham's paper is available on-line at: https://pubmed.ncbi.nlm.nih.gov/11696422/

Physical Activity, Obesity, Height, and the Risk of Pancreatic Cancer
- August 2001
In this week's issue of the Journal of American Medical Association (JAMA), Drs. Michaud, Giovannucci, Willett, Colditz, Stampfer, and Fuchs from the Harvard School of Public Health and the National Cancer Institute report a large study of pancreatic cancer risk. They used two large groups of patients - the Health Professions Follow-up Study and the Nurses Health Study to determine the risk factors of pancreatic cancer. A total of 46,648 men and 117,041 women were free prior cancer at baseline were followed for 10-20 years. Remarkably, the authors found that decreased physical activity, obesity, and being tall increased the risk of pancreatic cancer. The effect of increased physical activity on decreasing the risk of pancreatic cancer was the greatest among those who are overweight. The association between obesity and physical activity and the risk of pancreatic cancer was explained, biologically, by the association of abnormal glucose (sugar) tolerance and pancreatic cancer.
While additional studies are needed, this large epidemiologic study certainly suggests that increased physical activity and weight reduction are reasonable measures that may, in addition to reducing one's risk of heart disease, reduce the risk of pancreatic cancer.
Reference:
JAMA 286(8):921-929, 2001

Markers of Cancer Invasion
- July 2001
There is a great effort underway to identify new ways to identify cancers that otherwise would remain undetected for too long. A major approach is to identify tumor-specific markers and tissue-specific markers. For example, useful markers could be proteins found reproducibly to be produced by cancer cells in both tissue culture (cancer cells grown artificially outside the body) and in patient samples. But there is another potential type of marker, one that is produced by the invasive tumor or the body's reaction to it. Such markers would not be normally found in tissue culture and might not be present in normal tissues at a high level. These are the invasion-specific markers.
Dr. Ryu and colleagues in Dr. Kern's laboratory for pancreatic cancer research at Johns Hopkins searched for such genes. Dozens of invasion-specific markers were identified in invasive pancreatic cancers obtained from patient samples. Many of these were new markers not previously considered as cancer markers, and many of the genes are expressed not by the tumor cells but instead by the patient's response to the tumors. Some of these markers are known to be secreted and to be detectable in simple blood samples. A strong effort is underway to examine these candidates and develop markers for use in reliable assays for cancer that can be done on serum, to aid medical imaging, and to serve as targets for the development of invasion-specific anticancer therapy. The first such candidate marker was the subject of a recent paper by Argani and colleagues (See What's New, June 2001)
Reference:
Ryu, B., Jones, J., Hollingsworth, M. A., Hruban, R. H., and Kern, S. E. Invasion-specific genes in malignancy: Serial analysis of gene expression comparisons of primary and passaged cancers, Cancer Res. 61: 1833-1838, 2001. (abstract)

Activin Receptors — A New Anticancer Signal in Human Tumors
- June 2001
The major problem with human tumors is a social one. Tumor cells do not obey the signals from their surrounding cells that should restrain their growth. To date, very few of such signals have been defined, and this limits our ability to understand and counter this basic abnormality.
Because of the need to understand these signals, there has been a great effort to identify genes that are mutated and turned off in tumors. These are the tumor-suppressor genes. The inactivation of these genes allows tumors to escape from the normal growth controls that the surrounding cells and tissue are trying to place on them.
Activin is a protein secreted by normal cells. To exert its action, activin must bind receptors on a cell. The receptors propagate a signal to the cell, but it was not previously known that these signals were able to suppress tumor growth. Mutations within the activin receptor gene were found recently in some pancreatic cancers by Dr. Gloria Su and colleagues in Dr. Kern's laboratory for pancreatic cancer research at Johns Hopkins. In tumors that lack the mutations, someday it might be possible to administer activin as a therapeutic strategy. It might also be possible to mimic the effects of activin on tumor cells by a precise molecular targeting using specially designed new drugs that directly activate the signal pathway without the need for intact receptors. This is a new idea that was previously unknown, but now can be explored. It is our hope that one can design a rational therapy that would specifically attack the most vulnerable components of pancreatic cancer.
Reference:
Su, G. H., Bansal, R., Montgomery, E., Yeo, C. J., Hruban, R. H., and Kern, S. E. ACVR1B (ALK4) gene mutations in pancreatic carcinoma, Proc Natl Acad Sci USA. 98: 3254-3257, 2001.

Hopkins' Scientists use Molecular Tool to Discover New Markers of Pancreatic Cancer
- June 2001
In the June 1st issue of Cancer Research (https://aacrjournals.org/cancerres/article/61/11/4320/507556/Discovery-of-New-Markers-of-Cancer-through-Serial), Dr. Argani and colleagues from Johns Hopkins described the discovery of a new marker of pancreatic cancer. This new marker called "prostate stem-cell antigen" (PSCA) was discovered by using a technique developed at Johns Hopkins called "serial analysis of gene expression". Since the original description of SAGE, a group of cooperating scientists from a number of institutions have created an online database of gene expression that includes SAGE data on a variety of tissues and cancers. The investigators at Hopkins used this database to compare the gene expression levels in pancreatic cancer tissues with those seen in non-cancerous pancreatic tissues. The goal was to identify genes that were selectively "turned-on" in the cancers. One of the genes the Hopkins found using this approach coded for a protein called "prostate stem-cell antigen." Prostate stem-cell antigen is a gene that was originally thought to be largely restricted to prostate cells. Dr. Argani and colleagues demonstrate that prostate stem-cell antigen (PSCA) is, in fact, highly overexpressed in approximately 60% of primary pancreatic cancers. It is not expressed in the normal pancreas.
These findings are exciting for several reasons. First, they demonstrate the power of new technologies such as SAGE to discover new tumor markers. Second, PSCA, because it is selectively overexpressed in pancreatic cancers, maybe a useful marker for pancreatic cancer. Third, other groups have shown that PSCA can be an immune target and therefore PSCA is being explored as a target for the immune treatment of cancers. The demonstration of PSCA expression in pancreatic cancer suggests a new avenue for treating pancreatic cancers. That is, immunotherapy directed at cells expressing PSCA.
On an important side note, this work was supported, in large part, by generous donations from the friends and family of Michael Rolfe demonstrating the power of private giving to advance pancreatic cancer research.

Familial Pancreatic Cancer
- March 2001
For years, isolated reports in the medical literature have suggested that pancreatic cancer runs in some families. For example, it has been reported that former President Jimmy Carter lost his father, brother and two sisters from pancreatic cancer.
In the current issue of Clinical Cancer Research (https://aacrjournals.org/clincancerres/article/7/3/738/200023/Increased-Risk-of-Incident-Pancreatic-Cancer-Among), A. Tersmette and colleagues from Johns Hopkins report that first-degree relatives (brothers and sisters, parents and children) of patients with "familial pancreatic cancer" have a significantly increased risk of developing pancreatic cancer. Tersmette and colleagues followed 341 families enrolled in the National Familial Pancreas Tumor Registry (NFPTR) and found that the first-degree relatives of familial pancreatic patients had an 18-fold increased risk of developing pancreatic cancer when compared to the general population (the "SEER" database). In this study, familial pancreatic cancer was defined as at least a pair of first-degree relatives with pancreatic cancer in a family. Remarkably, if there were three or more family members with pancreatic cancer when the family enrolled in the NFPTR, then the risk of other family members developing pancreatic cancer jumped to 57-fold greater than the general population.
This study firmly establishes that "Familial Pancreatic Cancer" is a real entity and it provides a quantitative measure of the risk of pancreatic cancer in these families. Importantly, studies such as this will form the basis for identifying individuals at-risk for developing pancreatic cancer who might benefit from new screening tests as they are developed.
If you have a strong family history of pancreatic cancer and would like to join the research studies currently underway at Hopkins, please consider joining the NFPTR. If you would like to join, please contact the Coordinator of the NFPTR, Kieran Brune ([email protected]).

New Vaccine to Treat Pancreatic Cancer
- February 2001
In the January issue of The Journal of Clinical Oncology (volume 19; 2001: pages 145-156), Dr. Elizabeth Jaffee and colleagues at Johns Hopkins report the result of a phase I clinical trial of a novel vaccine treatment for patients with a pancreatic cancer. The vaccine was produced by genetically altering pancreatic cancer cells growing in culture so that the cells would produce large quantities of an immune activating factor called "Granulocytic-macrophage colony-stimulating factor" (or GM-CSF for short). Dr. Jaffee treated 14 patients with this vaccine in a phase I dose escalation trial. The patients underwent surgery at Hopkins after which they received various doses of the vaccine. No dose-limiting toxicities were encountered. Instead, Dr. Jaffee was able to demonstrate that the vaccine induced an anti-tumor immune response in three patients who received the highest dose of the vaccine (>10x107 vaccine cells). Remarkable, these three patients remained alive and free of disease more than 25 months after diagnosis. Based on these results, Dr. Jaffee and her team will be conducting phase II trials of the GM-CSF vaccine. These trials are scheduled to begin in the late spring - early summer.*
*In order to evaluate the effectiveness of the vaccine, a 60 patient study is planned to begin in May, 2001. As much as we would like to offer the vaccine to everyone, eligibility criteria had to be established for this study. Patients with adenocarcinoma of the pancreas who have surgery Johns Hopkins Hospital to remove their pancreas cancer and who have no clinical evidence of spread of the cancer outside the pancreas will be eligible for this study. Patients with bile duct cancer or neuroendocrine tumors or islet cell cancer are not eligible. Please contact Dr. Elizabeth Jaffee or Barbara Biedrzycki, R.N. ([email protected]) for more information on eligibility criteria.
2000 ▼
One Family Makes a Difference
- December 15, 2000
The family and friends of Michael Rolfe have turned their terrible loss into something wonderful. With vision, dedication, and hard work, the Michael Rolfe Research Foundation has raised over $250,000 for pancreatic cancer research at Johns Hopkins. The general support of the Foundation has led to the creation of a new laboratory for pancreatic cancer research at Johns Hopkins. This laboratory will be headed by Dr. Gloria Su and will be dedicated to developing a much-needed mouse model of pancreatic cancer. At the same time, the Michael Rolfe Research Foundation has had the vision to establish an endowment at Johns Hopkins. The interest income from this endowment will be used to support the Early Detection Laboratory at Johns Hopkins. Just as there is a PSA test for prostate cancer, so to is this laboratory dedicated to developing a test for the early detection of pancreatic cancer.
On November 30, 2000, Judy Rolfe, Lisa Rolfe Burik, James Rolfe, and Hazel Herzog visited the laboratory at Johns Hopkins and presented the pancreatic cancer researchers with a check for $100,000. This check represents the income from the 1st Annual Michael Rolfe Research Foundation Gala that was held in Highland Park, Illinois in August. Photographs of their visit to Hopkins which included the dedication of a plaque in honor of Michael Rolfe can be seen below. Click here for details about Michael Rolfe and the Michael Rolfe Research Foundation.
The generous support of the Michael Rolfe Research Foundation exemplifies how private donations can honor loved ones and, at the same time, have a significant impact on the war against pancreatic cancer research.



Hopkins' Scientist Isolate Genes from Tumor Vessels
- August 22, 2000
In an exciting advance which may lead to new cancer therapies, Dr. St. Croix in Bert Vogelstein and Ken Kinzler's laboratory at Johns Hopkins reported their analysis of blood vessels from normal and cancerous colon tissues in this week's Science (August 18, 2000: 1197-1202). Using a powerful new technique developed at Hopkins, called serial analysis of gene expression (SAGE), Dr. St. Croix and colleagues were able to identify 46 genes which appear to be specifically elevated (or "turned on") in tumor-associated blood vessels.
The identification of these genes is exciting because such genes could potentially be targets for new drugs aimed at shrinking tumors by starving them of their blood supply. Alternatively, these genes which are selectively "turned on" in the tumor blood vessels could be used to specifically target therapies to the cancer. Importantly, most of the genes they identified were also "turned on" in a wide range of different tumor types, including pancreatic cancer, suggesting that the findings of Dr. St. Croix and colleagues could be translated to pancreatic cancers.
Dr. St. Croix's paper is available on the Web at https://www. sciencemag.org/cgi/content/full/289/5482/1197
Reference:
St Croix, B., Rago, C., Velculescu, V., Traverso, G., Romans, K.E., Montgomery, E., Lal, A., Riggins, G.J., Lengauer, C., Vogelstein, B., and Kinzler, K.W. Genes expressed in human tumor endothelium. Science, 289: 1197-1202, 2000.

Kudos to Hopkins Team
- June 30, 2000
John L. Cameron, MD, The Alfred Blalock Professor and Chairman of the Department of Surgery at Hopkins has lead the pancreatic cancer surgery team here at Hopkins for several decades. We are pleased to announce that he has been elected president of the American Surgical Association. The association, established in 1878, is the oldest surgical association in the United States. The presidency of the ASA is regarded as the highest honor one can achieve in American surgery.
Bert Vogelstein, M.D., is the "father" of the modern cancer research effort at Hopkins. Bert is a pioneer of the idea that cancer is a genetic disease. His research focuses on colon cancer, but he he has trained much of, and collaborates extensively with, the pancreas cancer research team. We are pleased to announce that Bert received the General Motors Cancer Research Foundation Charles S. Mott Prize. The award, which includes a $250,000 prize, honors contributions to the discovery of cancer causes or prevention. Vogelstein received the award at a ceremony at the National Institutes of Health.

Finding a Diamond in the Gravel
- June 13, 2000
Some time ago, here in the "What's New" pages, we introduced an analogy. We noted that we get televisions fixed when only a single small part breaks. The same holds for cars, houses, and bicycles. Maybe if we knew just what was broke in a cancer cell, we could fix it.
But how? We've made great progress in understanding what is broke in cancer cells. Yet, how to fix them? Even if it were as easy as car repair, there are problems. When faced with a screw that needs a ratchet, we usually hunt and peck until the right fit is found. After a dozen tries, you are guaranteed to find the one. This may not be such a bad strategy to find the right fit of a drug to the problem of cancer.
In the recent issue of the journal, Cancer Research (June 15), Johns Hopkins researchers from the Kern Laboratory tried this very technique. Hopkins postdoctoral fellow Dr. Gloria Su and resident in surgery Dr. Taylor Sohn tried over 16 thousand chemical compounds, looking for ones that could turn on a specific function that is often broken in pancreatic cancer (e.g., the DPC4 signaling pathway that regulates cell growth). The process was done with very small volumes of cancer cells in culture - over 16 thousand small colonies of cells! Like sifting for diamonds in a pile of gravel, they had to use a special technology to screen through all the cultures, to each of which was added just one of the compounds. A half-dozen promising compounds were identified. One of the compounds turned out to be a novel and specific inhibitor of a protein called HDAC (histone deacetylase). HDAC normally helps to turn off the functioning (the transcription, or expression) of cellular genes. The new compound was termed Scriptaid, a new and more nontoxic member of a class of compounds that is currently under investigation for antitumor effects.
It is a major aim of the Hopkins research effort to expand out efforts in drug screening. We hope to find new approaches to fix the cancer cells by screening for compounds that do specific things, like turning on the broken pathways that normally would regulate the cells of the pancreas. These first attempts are exciting, but like diamonds in the rough, only hint at the gems to be uncovered.
Reference:
Su GH, Sohn TA, Ryu B, Kern SK. A novel histone deacetylase inhibitor identified by high-throughput transcriptional screening of a compound library. Cancer Res 60, 3137-3142, 2000.

Inactivation of the Breast Cancer Gene (BRCA2) in the Precursors to Invasive Pancreatic Cancer
- May 12, 2000
In this month's issue of the American Journal of Pathology, Dr. Michael Goggins and colleagues from Johns Hopkins report an exciting study of the timing of inactivation of the second breast cancer gene (BRCA2) in the early development of pancreatic cancer. Dr. Goggins had previously shown that ~7% of patients with pancreatic cancer, particularly those of Ashkenazi Jewish heritage, developed their cancer because they inherited a mutation in the second Breast Cancer Gene (BRCA2) (see Cancer Research 1996, volume 56, pages 5360-5364). This earlier finding by Dr. Goggins suggests that some patients with pancreatic cancer, particularly those with a strong history of breast cancer, may benefit from genetic testing for mutations in BRCA2.
In the current study, Dr. Goggins and colleagues looked at the timing of BRCA2 inactivation in early pancreatic cancers and they found that this inactivation occurs relatively late (biologically speaking). This important finding by Dr. Goggins and his team may explain why the pancreatic cancers which develop in patients with inherited mutations in BRCA2 arise late in life.
Also in this month's issue is a second article on pancreatic cancer from the Hopkins Scientists. In this article, Dr. Wilentz and colleagues further characterize a newly recognized form of pancreatic cancer called "medullary cancer."

Hopkins Scientists Make Two Advances Towards Early Detection of Pancreatic Cancer
- April 10, 2000
The April 1, 2000 issue of Cancer Research contains two reports on pancreatic cancer from scientists at Johns Hopkins. In the first of these two papers, Dr. Michael Goggins reports that multiple genes are "hypermethylated" in pancreatic cancer.
Methylation refers to the addition of an extra carbon atom to DNA and it is a common mechanism by which cancer preventing (tumor suppressor) genes are inactivated in cancers. Goggins' team studied a series of 45 pancreatic cancers and, remarkably, they detected hypermethylation in at least 60% of the cancers. Importantly, hypermethylated genes have been used in other organ systems to develop screening tests for cancer. The finding by Goggins and colleagues may help in the development of a new screening test for pancreatic cancer.
Also in the April 1, 2000 issue of Cancer Research, Dr. Robb Wilentz and colleagues from Johns Hopkins report detailed studies on the genetic alterations in precancerous lesions (called PanINs) in the pancreas. Wilentz et al. demonstrated that loss of the DPC4 tumor suppressor gene occurs late in the development of these precancerous lesions, suggesting that DPC4 loss could be a good marker for a high risk of developing an invasive cancer of the pancreas.
Taken together, these studies help define some of the genetic alterations which, in the future, may be used to develop new screening tests.
References:
Ueki, T., Toyota, M., Sohn, T.A., Yeo, C.J., Issa, J.-P.J., Hruban, R.H., and Goggins, M. Hypermethylation of multiple genes in pancreatic carcinoma. Cancer Res, 60: 1835-1839, 2000.
Wilentz, R.E., Iacobuzio-Donahue, C.A., Argani, P., McCarthy, D.M., Parsons, J.L., Yeo, C.J., Kern, S.E., and Hruban, R.H. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: Evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res, 60: 2002-2006, 2000.

Quality of Life and Outcomes After Pancreaticoduodenectomy
- February 11, 2000
Pancreaticoduodenectomy has been gaining acceptance as an appropriate procedure for various malignant and benign diseases in and around the pancreas. As experience with pancreaticoduodenectomy grows, there are increasing numbers of pancreaticoduodenectomy survivors who have recovered from their procedure and who live with the requisite altered upper gastrointestinal anatomy. This study represents the largest study ofquality of life in Whipple survivors reported to date. While there is a general impression that pancreaticoduodenectomy can severely impair quality of life and alter normal activities, the results of this study prove otherwise.
A standard quality of life questionnaire and functional outcomes survey was received back from 192 Whipple patients who were operated on at the Johns Hopkins Hospital between 1981 and 1997, inclusive. The quality of life survey assessed 30 items on a visual analog scale, categorized into three domains: physical, psychological, and social. Scores were reported as a percentile, with 100% being the highest possible score. The same quality of life questionnaire was also sent to laparoscopic cholecystectomy patients and to healthy individuals, both to serve as control groups.
The overall quality of life scores for the pancreaticoduodenectomy patients in the three domains (physical, psychological, social) were 78%, 79%, and 81%, respectively. These scores were comparable to those of laparoscopic cholecystectomy patients (scores of 83%, 82%, and 84%, respectively) and healthy controls (scores of 86%, 83%, and 83%, respectively). Assessment of post-pancreaticoduodenectomy functional outcomes indicated that some common problems included early weight loss, abdominal pain, fatigue, and loose stools.
These data provide the largest single institution experience assessing quality of life after the Whipple procedure. Remarkably, these data demonstrate that surviving Whipple patients as a group have near normal quality of life scores. This corrects the general misimpression that Whipple patients have severely impaired quality of life, and cannot return towards normal activities.

Immunohistochemical Staining for DPC4: From Gene Discovery to Clinical Application
- January 10, 2000
In 1996, Dr. Scott Kern and colleagues at Hopkins discovered a new gene which appeared to be selectively inactivated (deleted) in the development of cancer of the pancreas. Dr. Kern and his colleagues named this gene "DPC4" for Deleted in Pancreas Cancer locus 4 (see Science 1996, vol. 271:350-353). The discovery of this gene was exciting because DPC4 is inactivated in a large number of cancers of the pancreas, but also because its inactivation appears to be relatively specific for cancer of the pancreas. That is, DPC4 appears to be only rarely inactivated in other tumor types.
In addition to adding to our understanding of the basic biology of pancreatic cancer, the discovery of the DPC4 gene by the Hopkins team has a number of important clinical applications. For example, in this month's issue of The American Journal of Pathology (Am J Pathol 2000, vol 156:37-43), Robb Wilentz and colleagues from Johns Hopkins report a new immunohistochemical stain for DPC4. This new stain can detect DPC4 in tissue sections (such as biopsies), and the staining pattern directly mirrors the DPC4 gene status. Because of its simplicity and availability, the immunohistochemical staining technique Dr. Wilentz developed for DPC4 has a number of direct clinical applications. For example, staining for DPC4 may help to distinguish benign chronic pancreatitis (which should express DPC4) from cancer of the pancreas (half of which will not express DPC4). Thus, DPC4 staining will add a valuable tool to the interpretation of needle biopsies of the pancreas. In addition, the immunohistochemical assay reported by Wilentz and colleagues for DPC4 may lead to answers in the investigative area. For instance, the immunohistochemical study of early lesions in the pancreas may help determine the stage at which DPC4 inactivation contributes to neoplastic progression and thereby help in the development of new screening tests for early pancreatic cancer.
1999 ▼
Mucinous Cystic Tumors of the Pancreas
- November 19, 1999
Mucinous cystic tumors are rare neoplasms of the pancreas characterized by the presence of large cysts (fluid filled cavities) lined by mucin producing cells. Some investigators have suggested that all mucinous cystic neoplasms are malignant (capable of spreading to other organs) and that all mucinous cystic tumors should therefore be designated as cancers "mucinous cystadenocarcinomas."
Investigators at Johns Hopkins studied over 60 of these rare tumors and found that with careful examination they could accurately separate mucinous cystic tumors into two groups those that are entirely benign (the tumors never recurred) if completly resected ("mucinous cystadenomas") and those that had a malignant or cancerous potential ("mucinous cystadenocarcinomas"). This study by Dr. Robb Wilentz is reported in the November issue of the American Journal of Surgical Pathology (volume 23, pages 1320-1327). Importantly, two-thirds of the mucinous cystic tumors in Dr. Wilentzs study fell into the entirely benign group. This means that simply lumping all mucinous cystic tumors into the malignant category would have incorrectly labeled two-thirds of the patients as having cancer when they didnt!
The study is also important because it highlights the impact private donations can have on research. Dr. Wilentz conducted this research during a research fellowship year he spent in the laboratory of Dr. S. Kern and this fellowship was supported by the Helen S. Heller and Daniel Kim Memorial Funds for pancreatic cancer research at Hopkins. Without this private support, Dr. Wilentz would not have been able to do his study. Private giving makes a difference!
Reference:
Wilentz RE, Albores-Saavedra J, Zahurak M, Talamini MA, Yeo CJ, Cameron JL, Hruban RH. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol 23(11):1320-1327, 1999.

Peutz-Jeghers Gene Shown To Play a Role in the Development of Pancreas Cancer
- June 23, 1999
Twelve years ago doctors at Johns Hopkins reported that patients with a rare syndrome, called "The Peutz-Jeghers Syndrome," had an increased risk of developing pancreatic cancer.1 The reason for this increased risk remained a mystery until now.
The Peutz-Jeghers Syndrome is a rare inherited syndrome in which affected patients develop dark pigmented spots on their lips ("mucocutaneous melanin macules") and polyps in their intestinal tract ("hamartomas"). These patients have an increased risk of cancer, especially pancreatic cancer. In the June 1999 issue of the American Journal of Pathology, Gloria Su, Ph.D. and colleagues from Johns Hopkins explain this association.2
The STK11/LKB1 gene in chromosome 19 is responsible for the Peutz-Jeghers Syndrome. Gloria Su and colleagues examined the status of the STK11/LKB1 gene in a large series of pancreatic cancers and in pancreatic cancer resected from patients with the Peutz-Jeghers Syndrome. They found that the STK11/LKB1 gene was inactivated in 4-6% of the pancreatic cancers. While the inactivation of this gene plays a role in only a small percentage of pancreatic cancers, Gloria Su and colleagues made a second, quite remarkable discovery. They found that the inactivation of the STK11/LKB1 gene in patients with the Peutz-Jeghers Syndrome explained the development of pancreatic cancer in these patients. Thus, a long-standing mystery was solved. The inheritance of a defective copy of the STK11/LKB1 gene causes the Peutz-Jeghers Syndrome and the inactivation of this gene in these patients explains their increased risk of pancreatic cancer.
Reference:
Giardello FM, Welsh SB, Hamilton SR, Offerhaus GJA, Gittelsohn AM, Booker SV, Krush AJ, Yardley JH, Luk GD: Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med 1987, 316:1511-1514.
Su GH, Hruban RH, Bansal RK, Bova GS, Tang DT, Shekher MC, Westerman AM, Entius MM, Goggins M, Yeo CJ, Kern SE. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol 154(6):1835-1840, 1999.

Standard vs. Radical Whipple
- May 28, 1999
Surgical resection is currently the most effective treatment for cancer of the pancreas. The extent of the surgery which should be performed is, however, controversial. Some have argued that the standard pancreaticoduodenectomy (Whipple procedure) should be extended to include the removal of the distal stomach (distal gastrectomy) as well as the removal of additional lymph nodes (retroperitoneal lymphadenectomy). The more extended surgery is called a "radical pancreaticoduodenectomy". The controversy over standard versus radical Whipple has been difficult to resolve, because most centers do one surgery or the other and data between institutions may not be comparable.
In the May 1999 issue of the Annals of Surgery, Drs. Yeo, Cameron and colleagues from Johns Hopkins report a study that may finally help resolve this controversy. They report a randomized single-institution trial in which patients were randomized to receive either a standard or a radical Whipple. Of the 114 patients randomized, 56 underwent a standard Whipple and 58 a radical Whipple. Dr. Yeo and colleagues found that the two procedures can be performed with similar morbidity and mortality. Importantly, the one-year survival rate for both groups was similar (~80%).
This important study will continue and the patients enrolled will be followed to determine if there are any long-term benefits to doing a radical Whipple. For now, part of the controversy in the radical versus standard Whipple debate has been answered. Both can be performed with equal morbidity and mortality, but the radical Whipple does not provide any improvement in survival at one year.
Reference:
Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, Pitt HA, Lillemoe KD. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma. Comparison of morbidity and mortality and short-term outcome. Annals of Surgery 1999; 229: 613- 624.

Robb Wilentz Wins an Award
- March 26, 1999
Robb Wilentz, one of the pancreas cancer research fellows at Johns Hopkins, won the 1999 Gastrointestinal Pathology Society Award for best presentation by a resident or fellow at the 1999 meetings of the United States and Canadian Academy of Pathology. Robb won the award for his presentation entitled "Morphology Accurately Predicts Behavior of Mucinous Cystic Neoplasms of the Pancreas" (Modern Pathology, Volume 12, 1999, Page 169A). This work is important because it has been previously suggested that all cystic tumors of the pancreas which produce mucin (so called mucinous cystic neoplasms) are malignant (cancerous). Robb showed that a careful microscopic examination of these tumors can be used to separate them into two groups: those patients who are completely cured if their tumors are removed surgically and those patients who need additional therapy. Using the techniques Robb outlined a group of patients with these tumors can be told that they are cured while other patients will receive the benefit of additional therapies. Our congratulations go out to Robb for a job well done.
Reference:
Wilentz et al. Morphology Accurately Predicts Behavior of Mucinous Cystic Neoplasms of the Pancreas. Modern Pathol 12(1):169A, 1999.

More on "What's Broke in Pancreatic Cancer?"
- March 1, 1999
A few months ago here in the What's New pages, we introduced an analogy. We noted that we get televisions fixed when only a single small part breaks. The same holds for cars, houses, and bicycles. Maybe if we knew just what was broken in a cancer cell, we could fix it.
We continue to make progress in figuring out "What's Broke." In a study published recently (Cancer Research 58:9329), additional clues implicate an important tumor suppressor system. This study was led by Michael Goggins, a postdoctoral fellow in the Kern laboratory and now a new faculty member (see below for the establishment of his new laboratory dedicated to developing screening techniques for the early diagnosis of pancreatic cancer). He discovered that some pancreatic and biliary cancers have mutations or deletions of certain genes that produce proteins for the surface of the pancreatic cells. One of the most well-studied proteins in cancer research is one called TGF-beta. TGF-beta exists outside of cells, in tissue and circulating in blood, but can bind to receptors on a cell's surface. When this happens, the cell is instructed to slow its growth, or to become a more mature type of cells (called "differentiation"), or even for the cell to cease living (called "apoptosis", from a Greek word that refers to the falling off of leaves from trees in the fall). Cancer cells are notoriously poor in responding normally to TGF-beta's suppressive effect, but exactly why has been difficult to figure out. Mutations in the receptors are only rarely found. Dr. Goggins' study was the first ever to show that cancers can genetically inactivate both of the major types of TGF-beta receptors. This study also provided the first concrete evidence that pancreatic cancers arise, in part, because they acquire the ability to evade the normal control of TGF-beta.
In a related discovery published last month (Proc Natl Acad Sci USA96:1427), Dr. Jiale Dai, another postdoctoral fellow in the Kern laboratory, helped us understand the role of another tumor-suppressor of pancreatic cancer. The DPC4 gene is mutated or is deleted in just over half of pancreatic cancers. No other tumor type has as many alterations in DPC4 as does pancreatic cancer, suggesting that the study of DPC4 may hold some important lessons for pancreatic cancer. Dr. Dai discovered that if he took cells that made no DPC4, and then genetically engineered them so they would produce DPC4, the cells grew much slower. This is what you might expect for a tumor-suppressor gene. He then expanded on these findings in a clever way. He made a special form of DPC4 where he could control its activity at will. Upon adding a certain chemical, he could make the DPC4 go from an inactive form to an active form within minutes. By doing this type of comparison, he found that active DPC4 could kill the cancer cells, and it seemed that this may have been the major way by which DPC4 acts as a suppressor gene. When he studied a mutant form of DPC4 found in a patient's cancer, the opposite results were found. Now instead of suppressing growth and causing cell death, growth was stimulated and the cancer cells were protected from death.
Due to Dr. Dai's work, we now have a clearer understanding of just how pancreas cancer cells come to misbehave. Now that he knows the overall effects of DPC4, Dr. Dai is currently refining his studies to understand the exact mechanism by which DPC4 accomplishes it all.
These and future research efforts should help us to understand "What's Broke" in pancreatic cancer. Only then will we be empowered to fix it.


New Laboratory Established at Hopkins for the Early Detection of Pancreatic Cancer
- February 2, 1999
All too often patients with pancreas cancer do not come for medical attention until after the disease has spread beyond the pancreas. We urgently need a new test for the early diagnosis of pancreatic cancer, so that these cancers can be detected at their earliest stages while they are still curable. Johns Hopkins is therefore pleased to announce that on February 1, 1999 we opened a new laboratory dedicated to finding new methods to detect early pancreatic cancer. Dr. Michael Goggins will head this laboratory. Remarkably, the creation of this lab was made possible by funds raised from the November 8th, 1998 Pancreatic Cancer Fundraiser held in California, and by generosity of users of this Web page! The creation of this lab shows what "people power" can accomplish.
Number one on the list of goals for this laboratory is to develop a screening test for early pancreatic cancer. Screening tests for breast cancer, colon cancer, and prostate cancer already exist and have helped save lives. Dr. Goggins has dedicated his laboratory effort to finding the "PSA test for pancreatic cancer."
Although we were able to raise enough funds to open this exciting new laboratory, we have only raised approximately $100,000 of the $400,000 needed to operate the laboratory over the next three years.
1998 ▼
Some Thoughts on Rational Therapy for Pancreatic Cancer
- November 4, 1998
"If something's broke, can't you fix it?"
Although we don't generally think of them this way, cancer cells have a lot in common with normal cells. Kind of like the "good kid" that commits some horrendous crime. That's why we can't sweep the criminals off the streets, and why we can't easily imagine powerful cancer drugs that do their magic without any unintended side effects. In all likelihood, most of the rules a cancer cell lives by, are the same rules the other cells in your body live by. This makes cancer therapy hard.
But certainly, this can't be right. We get televisions fixed when only a single small part breaks. The same holds for cars, houses, and bicycles. Maybe if we exactly just what was broke in a cancer cell, we could fix it. This is the goal known as the search for "rational therapy". Many people don't realize it, but virtually none of our current anticancer drugs were originally designed to cure cancer. They were, in actuality, found by chance, often through the screening of tens of thousands of compounds to see which ones happened to work. It is no wonder that they are a disappointing answer to the cancer problem.
But this analogy still has problems. What if your auto mechanic knew exactly the part needed for your car, but he couldn't order it because no one made the spare parts for your vehicle? And this is why rational therapy in cancer is not currently available. The parts do not yet exist. Although the genetic revolution in cancer research has shown us what parts are missing, more efforts need to be devoted to design those spare parts and the tools needed to install them.
A major step towards this is now underway for pancreatic cancer. Drug companies have been trying to develop drugs that turn off the overactive mutant K-ras gene that exists in over 90% of pancreatic cancers, so far without success, but with continued hope. Recently, Dr. Kern's laboratory at Hopkins has published its studies that begin to build a foundation for rational therapy based on the tumor-suppressor gene, DPC4.
First they needed to know what was "broken" in pancreatic cancer. DPC4 helps receive and then implement a suppressive signal that controls the behavior of cells. But how it did that was unknown. Jiale Dai, a postdoc in the laboratory, discovered that the DPC4 could bind to specific sequences of DNA. This was published last March (Molecular Cell 1998, 1:611). That paper outlined how DPC4 can identify certain locations on DNA. Once it is attached to DNA at these sites, it stimulates the production of protein from the nearby genes. These proteins then presumably cause the cell to slow its growth, causing an important tumor-suppressive effect and preventing the cell from growing like a cancer. This effect is lost when DPC4 gets deleted or mutated in many pancreatic cancers.
In follow-up work just published this month (Cancer Research 1998, 58:4592), Dr. Dai presented a comprehensive study of these small mutations in DPC4. He was able to classify the various mutations into three types. One type directly interferes with the ability of DPC4 to bind to DNA. Another class of mutations allows DPC4 to bind to DNA, but interferes with its ability to increase the production of the nearby genes. A third type of mutation interfered with the ability of DPC4 to receive the suppressive signals that it is supposed to help transmit. Indeed, even when DPC4 is not lost or mutant (which is the case in half of pancreatic cancers), there is often a weakness in this original signal. Normally, these "upstream" signals cause DPC4 to move from the outer part of the cell (the cytoplasm) to the nucleus of the cell, where DNA and genes are located.
Dr. Dai then proposed a therapeutic strategy, a completely new drug design that could act to help overcome some of these problems. He proposed that a drug which attached to DPC4 and caused it to relocate to the nucleus might augment any defective upstream signals, potentially aiding the cell's own tumor suppression system and specifically overcoming some of the cancer defects. He then tested some newly-engineered forms of DPC4 protein that he could send to the nucleus at will. These were able to enhance the weakened signals of some pancreatic cancer cells, and even returned some function to mutant DPC4 proteins (those in the third class described above). This is the first experimental evaluation of a designer drug strategy to turn on the broken tumor-suppressor genes in pancreatic cancer.
The science of the new millennium will admit its shortcomings if its promising ideas do not at first produce fruit. But the search for rational drug design will persevere. This research will be exciting, expanding in scope and sophistication. We will tackle cancer without leaving the task to chance alone.

New Website Section: Frequently Asked Questions About PC
- October 5, 1998
We are creating a new section for this web site! This section will cover Frequently Asked Questions about PC. It is currently being designed and will be added to this site in early 1999. We want this to be a useful resource for new visitors as well as for the repeat visitors that have been dealing with PC for some time.
I am asking for your suggestions for Frequently Asked Questions. What are the questions you had or still have in dealing with this disease? I am particularly interested in those questions that will benefit from a visual explanation (a drawing, photograph, diagram, chart, list, animation, etc.).
You may want to respond in a "Top Ten" format with #1 being the most urgent or confusing topic. This will give me a good sense of what is most important to PC patients, family and friends. The questions could be related to the anatomy or function of the pancreas, symptoms, treatments, surgery, adjuvant therapy, post-operative care, genetics, metastases, etc.
Please don't hesitate to include ANY question or suggestion. Think of this as a virtual brainstorming session. You can remain anonymous if you want and your suggestions will be confidential, or feel free to include a return e-mail address. I will be collecting suggestions over the next month.
WHO AM I? Jennifer Parsons, a graduate student in Medical Illustration at Johns Hopkins School of Medicine, pursuing this project as my Masters thesis.
The preceptor for my thesis is Dr. Hruban, Director of the National Familial Pancreas Tumor Registry. I will be working with him and the rest of this site's planning committee (listed under Clinicians/Researchers) over the next six months.

Significant Decrease In Hospital Mortality Rate In Maryland Associated With Regionalization of the Surgery
- August 7, 1998
In the July, 1998 issue of the Annals of Surgery, Toby Gordon ScD and colleagues examined the statewide in-hospital mortality rate for pancreaticoduodenectomies (Whipple resections) performed in Maryland over a 12-year period. A total of 795 pancreaticoduodenectomies were performed in Maryland at 43 hospitals from 1984 to 1995. During this period, one institution (Johns Hopkins) increased its yearly share of pancreaticoduodenectomies from 20.7% to 58.5%. This increase in regionalization of the surgery (performance of the surgery at specialized high volume centers such as Hopkins) was associated with a remarkable decline in the statewide in-hospital mortality rate for the procedure from 17.2% to 4.9%. After adjustment for patient characteristics and study year, hospital share (the number of Whipples the hospital performs per year) remained a significant predictor of mortality. Remarkably, an estimated 61% of a decline in the statewide in-hospital mortality rate for pancreas cancer surgery was attributable to the increase in share of surgeries performed at the high volume provider (Hopkins). These data confirm previous observations by Toby Gordon and others that suggest that pancreaticoduodenectomies (Whipple procedures) are safest when they are performed at hospitals with extensive experience with this procedure, and which perform a large number of Whipples every year. Furthermore, the data suggest that as the health care market is restructured, that regionalization of health care services should result in optimization of outcomes for complex, high risk elective surgeries.
Reference:
Gordon TA; Bowman HM; Tielsch JM; Bass EB; Burleyson GP;Cameron JL. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Annals of Surgery 228: July 1998, pages 71-78.

The MKK4 Gene and Pancreas Cancer
- July 22, 1998
When is an accelerator used as a brake?
When it's a new type of tumor-suppressor gene, apparently.
Cancer cells could be said to be hyperactive. They make new cells faster than is appropriate, and invade neighboring areas when they should stay still. Cancer cells have two major types of changes in their genes that cause this. Some of the normal growth-promoting genes become overactive, and these genes are termed oncogenes. In the classic automotive metaphor, they correspond to the cell's accelerator pedal. A separate set of genes normally suppresses the cell, and are called tumor-suppressive genes. These are the brakes. When oncogenes are activated, or tumor-suppressor genes are turned off, tumors can grow and spread. Thus in a cancer cell, genetic changes are easy to interpret. If a change appears to activate a gene, the gene is an oncogene. If it inhibits or deleted a gene, the gene is a tumor-suppressor gene. Easy.
The process by which one cell becomes two is called "mitosis". Some of the substances that a cell encounters in its environment act to stimulate the cell to undergo mitosis. These are called, "mitogens". Once a cell encounters a mitogen, a number of proteins in the cell relay the signal down to the nucleus of the cells where it is interpreted as a command to grow and divide, forming new cells. These proteins are the subject of a great deal of scientific interest, and are in a category of proteins called, "mitogen-activated protein kinases", or simply "MAP kinases". In a cancer cell, MAP kinases were initially expected to be activated - certainly not inactivated. MAP kinases were interesting in part because they are suspected of acting as oncogenes.
Eyebrows were raised when the first genetic changes were found in a human MAPK gene. Myriad Genetics reported finding occasional alterations in a gene called MKK4, or "MAP kinase kinase 4". The mutations were rare, affecting only a few percent of a very large panel of samples that consisted of multiple tumor types. Mutations were found in two pancreatic cancers, as well as some tumors of testis, breast, and colon. And the mutations included inactivating mutations and total deletions of the gene, clearly suggesting that MKK4 was a tumor-suppressor, not an oncogene! Perhaps because this was a very confusing set of findings, the discovery did not seem to generate the excitement that some felt was due.
Dr. Gloria Su, as postdoctoral fellow of the Kern laboratory at Johns Hopkins, has now published the first study that confirms these findings. MKK4 indeed harbors the classic mutations of a tumor-suppressor gene. In her recent study, published in the June 1 issue of the journal Cancer Research, mutations were found to affect about 4% of pancreatic cancers, some biliary cancers, and 15% of breast cancers. This makes MKK4 the seventh tumor-suppressor gene found to involve pancreatic cancer, and one of the more common tumor-suppressor genes mutated in breast cancer. Her study also determined for the first time that the mutations indeed occurred during the growth of the tumors.
A closer look at MKK4 shows that it is much more than a simple mitogenic gene. For the pathways regulated by MKK4 are also activated by stresses, such as exposure to chemotherapy. The MKK4 pathway appears to be responsible for a number of consequences, such as cell death and differentiation (moving the cell toward a more mature, functioning state), that indeed do make sense for a tumor-suppressor gene to participate in.
The low percentage of mutations raises a number of questions. Are there other important genes of the MKK4 pathway that are mutated at a high frequency, but that have been simply overlooked? Can we find a way to use the pathway in diagnosis? If the pathway is important enough to be mutated in some patients, but still remains intact in most cancers, can it be harnessed for therapy of the disease? What exactly does the pathway do in pancreatic cells? How soon until we understand why it is important in tumorigenesis?
The discoveries will come in time. The pursuit for these answers is what keeps pancreatic cancer research so exciting.
Reference:
Cancer Research 1998; 58:2339-2342

Whipple Resections for Cancers in the Area of the Head of the Pancreas
- July 9, 1998
Much of the survival data for patients who undergo Whipple resections (pancreaticoduodenectomies) for cancers of the pancreas, duodenum, bile duct, or ampulla vater, are based on short-term survival data. There is very little data on long-term follow-up of patients who have undergone a pancreaticoduodenectomy for cancer. In the June issue of the Annals of Surgery, Dr. Yeo and colleagues (see reference) reviewed the Johns Hopkins experience with patients treated five or more years ago. Unlike previous series which have used predicted ("actuarial") five-year survival data, Dr. Yeo and colleagues look at actual five-year survival rates in these patients.
From April of 1970 to May, 1992, 242 patients underwent a Whipple resection for a periampullary carcinoma at the Johns Hopkins Hospital. Follow-up was complete through May of 1997, and actual five-year survival rates were calculated. Of the 242 patients with resected cancers, 149 of the cancers arose in the pancreas, 46 arose in the ampulla vater, 30 in the distal bile duct, and 17 in the duodenum. There was a 2% operative mortality in the last 100 patients. There were 58 five-year survivors, 28 seven-year survivors, and 7 ten-year survivors. Predictors of five-year survival in this group of patients included well differentiation of the tumor, negative resection margin, and negative lymph node status (no metastases to the lymph nodes). In addition, the site of origin of the cancer predicted survival: 15% of the patients with pancreatic cancers survived five years; 39% of the patients with ampullary cancers survived five years; 27% of the patients with bile duct cancers survived five years, and; 59% of the patients with duodenal cancers survived five years. From this analysis, Dr. Yeo and colleagues conclude that among patients with periampullary adenocarcinomas treated by Whipple resection, those with duodenal cancers are most likely to survive long term. In addition, resection margin status, resected lymph node status, and degree of tumor differentiation also significantly influence long-term outcome.
Reference:
Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe K, Pitt H. Periampullary adenocarcinoma. Analysis of 5-year survivors. Ann Surg 227:821-831 (1998).

Should Pancreaticoduodenectomy Be Performed In Octogenarians?
- May 27, 1998
The question often comes up, "How old is too old to have a Whipple?" In this month's issue of the Journal of Gastrointestinal Surgery (J Gastroentest Surg 1998;2:207-216) Drs. Sohn, Yeo, and colleagues, from Johns Hopkins, try to answer this question. They studied forty-six patients over the age of eighty who had a pancreaticoduodenectomy (Whipple resection) at Johns Hopkins. They then compared the outcome of these forty-six patients to the outcome of six hundred eighty-one patients who had a Whipple at Hopkins but were younger than eighty years of age. Interestingly, patients eighty years of age or older had a shorter operative time (6.4 hours versus 7 hours) but a longer post-operative length of stay (median fifteen days versus thirteen days). There were slightly more complications in the older patients than in the younger patients, but the mortality from the surgery was the same. Importantly, long-term survival was good for patients eighty years of age or older. The patients in the eighty years or older age group had an average survival of thirty-two months, and a five-year-survival rate of 19%. This compared to an average survival of twenty months and a five-year-survival rate of 27% in the patients younger than eighty years.
These data demonstrate that Whipple procedures (pancreaticoduodenectomies) can be performed safely in selected patients eighty years of age or older, with morbidity and mortality rates approaching those observed in younger patients. Based on these data, Dr. Sohn and colleagues suggest that age alone should not be a contraindication to pancreaticoduodenectomy.
Reference:
Sohn TA, Yeo CJ, Cameron JL, Talamini MA, Hruban RH, Sauter PA, Coleman J, Ord SE, Grochow LB, Abrams RA, Pitt HA. Should pancreaticoduodenectomy be performed in octogenarians? J. Gastroentest Surg 1998;2:207-216.

National Familial Pancreas Tumor Registry
- May 6, 1998
The National Familial Pancreas Tumor Registry (NFPTR) at Johns Hopkins is now 4 years old and over 300 families have joined this Registry. We periodically update the registrants with the latest development in pancreatic cancer research and I wanted to take this opportunity to share the letter we are sending out to the participants in NFPTR with you. Below is our spring 1998 letter.
Dear Friends of The NFPTR:
The last several years have witnessed dramatic advances in our understanding of the genetic basis for the development of pancreatic cancer and I wanted to update everyone who has helped our efforts with The National Familial Pancreas Tumor Registry (NFPTR).
The NFPTR has enrolled over 300 families (called kindreds). Slightly more than 130 of these kindreds are families in which two or more first-degree relatives have been diagnosed with pancreatic cancer, called "familial" cases. The remaining 170 families have only one family member with pancreatic cancer; these are called "sporadic" cases. Our initial analysis of the first 212 families (80 familial, 132 sporadic) enrolled in The NFPTR revealed that the increased risk of pancreatic cancer seen in familial cases of pancreatic cancer may also extend to involve the second-degree relatives (aunts, uncles, cousins, etc.) of patients with pancreatic cancer. Twelve (3.7%) of the 324 second-degree relatives of the familial cases developed pancreatic carcinoma compared to only four (0.6%) of the 702 second-degree relatives of sporadic pancreatic cancer cases (See reference #1). Furthermore, this increased risk of cancer in second-degree relatives of familial pancreatic cancer cases was also seen in other cancer types (27.2% vs. 12.1%). The most common other cancers to occur in relatives of pancreatic cancer patients in this study were breast cancer, lung cancer, and colon cancer. These exciting preliminary results help establish that pancreatic cancer does indeed aggregate in some families. It is our hope that an understanding of the genetics pancreatic cancer will help define the causes of this aggregation. We have therefore concentrated our research efforts on understanding the genetics of pancreatic cancer. These genetic studies have been very fruitful and I wanted to update you on a few of our recent discoveries. We have:
- Discovered the Deleted in Pancreas Cancer 4 gene (DPC4 ) (reference #2) and showed that this gene is inactivated in approximately 50% of cancers of the pancreas. The identification of this gene may lead to novel therapeutic strategies to treat pancreas cancer.
- Helped in the discovery of the second breast cancer gene (BRCA2) (reference #3) and showed that this gene plays a role in the development of carcinoma of the pancreas (reference #4). Furthermore, it appears that patients who inherit a defective copy of this gene are at increased risk for developing pancreatic cancer. Our findings therefore suggest that patients with inherited BRCA2 gene mutations may benefit from screening both for breast cancer and for pancreatic cancer.
- Demonstrated that the p16 gene is frequently inactivated in pancreatic cancer (reference #5) and that some cases of familial pancreatic cancer are caused by inherited mutations (changes in the DNA sequence) in this gene (reference #6). Again, this finding has important implications for screening for early pancreatic cancers in patients with inherited p16 mutations.
- Described a new variant of pancreatic cancer. This new form can be recognized by routine microscopic examination. It is also associated with a specific genetic alteration called "microsatellite instability" (reference #7). This variant may have a better prognosis and may respond differently to chemotherapy and it is therefore important to recognize.
- Identified hundreds of genes overexpressed in pancreas cancer using a revolutionary new technique developed here at Johns Hopkins called SAGE (reference #8). We then demonstrated that these overexpressed genes can be used to develop new blood markers that are increased in persons with pancreatic cancer (reference #9). It is our belief that this approach might lead to the development of a new blood test that would enable the earlier diagnosis of pancreatic cancer.
- Demonstrated that many infiltrating carcinomas of the pancreas arise from microscopically identifiable precursor lesions in the pancreas (reference #10). This finding suggests that if these early lesions can be detected, patients can be treated before the cancer has spread beyond the pancreas.
- Characterized the genetic alterations in pancreatic cancer at the chromosome level and at the DNA level (references #11 and 12).
- Demonstrated that genetic alterations in pancreatic cancers can be detected in various other specimens such as duodenal fluid (reference #13) and in the stool (reference #14) of patients with pancreatic cancer. These studies established that an improved understanding of the genetic alterations of cancer of the pancreas may lead to new techniques to detect these tumors earlier.
- Demonstrated that the p53 tumor suppressor gene is frequently inactivated in pancreatic cancer (reference #15).
- We established a World Wide Web page on carcinoma of the pancreas. The Web address is https://pathology.jhu.edu/pancreas We are particularly proud of this Web page and we feel that it has provided an invaluable resource to patients, family members, and friends battling pancreatic cancer. For example, there have already been close to 1 million accesses to the Web page and over 15,000 messages have been posted in the chat room section of this Web page. These numbers are quite remarkable considering that approximately 27,000 Americans are diagnosed every year with pancreatic cancer. We continually update this Web page. For example, there is a section of this Web page entitled, "What's New" in which we post our latest discoveries. Those of you who have not visited this Web page are encouraged to do so.
- In addition, Dr. E. Jaffee here at Johns Hopkins has developed a pancreas cancer vaccine. This is a way to help a patient's own immune system battle against the cancer. Dr. Jaffee tells us that this vaccine has just completed its phase I clinical trials. She is in the process of analyzing the data and hopes to begin the phase II trial shortly. If successful, this vaccine will open up an entire new way to treat pancreatic cancer.
These are just some of our accomplishments over the last three to four years. A more complete list can be found in the References section of our site.
As you can tell from this summary, our work centers on the realization that cancer is a genetic disease. We believe that by studying families in which there is an aggregation of pancreas cancer and by studying the genetic alterations found in the cancers of the pancreas, we will better understand what causes it. Once we identify the causes we can develop new strategies to prevent this disease. Furthermore, once we identify the genes involved in the development of cancer of the pancreas, we can develop new gene-based screening tests to screen family members at risk for the disease. The goal of this would be to detect clinically early cancers while they are still surgically treatable. Finally, as we come to a better understanding of the genetics of cancer of the pancreas we can develop rational therapeutic strategies to treat this disease.
Finally, before I close, I wanted to let everyone know about an exciting fund raiser that Michael Landon Jr., (the son of the late TV actor) and Pam Acosta (one of the more active participants in our Web page) are organizing to raise awareness of carcinoma of the pancreas and to support our research efforts here at Johns Hopkins. This exciting dinner will take place on November 8, 1998 at The Beverly Hills Hotel in Beverly Hills California. Those of you wishing to find out more about this may contact Pam Acosta (909) 983-0655 or Deb Barbara at (410) 955-9485.
I sincerely wish everyone well and I hope that you find this information helpful.
With warm regards,
Ralph H. Hruban, M.D.
Associate Professor of Pathology
Associate Professor of Oncology
Director, National Familial Pancreas Tumor Registry
P.S. A number of you have asked about the results of any research that may have been done on your blood sample. These analyses take many years and in most cases we have not found any changes. Nonetheless, I wanted to assure you that we will contact you if we do find something that we feel may benefit your family.
Reference:
Hruban RH, Petersen GM, Kern SE. Genetics of Pancreatic Cancer. From Genes to Families. Surgical Oncology Clinics of North America 7:1-23, 1998.
Hahn SA, Schutte M, Hoque ATMS, Moskaluk CA, daCosta LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor-suppressor gene at human chromosome 18q21.1. Science 271:350-353, 1996.
Schutte M, da Costa LT, Hahn SA, Moskaluk C, Hoque ATMS, Rozenblum E, Weinstein CL, Bittner M, Meltzer PS, Trent JM, Yeo CJ, Hruban RH, Kern SE. Identification by representational difference analysis of a homozygous deletion in pancreatic carcinoma that lies within the BRCA2 region. Proc Natl Acad Sci USA 92:5950-5954, 1995.
Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein C, Petersen GM, Yeo CJ, Jackson CE, Lynch HT, Hruban RH, Kern SE. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Research 56:5360-5364, 1996.
Schutte M, Hruban RH, Geradts J, Maynard R, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schmiegel W, Baylin SB, Kern SE, Herman JG. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Research 57:3126-3130, 1997.
Moskaluk CA, Hruban RH, Lietman A, Jackson C, Yeo CJ, Lynch HT, Kern SE. Novel germline p16INK4 mutations in familial pancreatic carcinoma. Human Mutation (in press, 1998).
Goggins M, Griffin CA, Turnacioglu K, Hilgers W, Hahn SA, Shekher M, Tang D, Song T, Offerhaus J, Yeo, CJ, Kern SE, Hruban RH. Adenocarcinomas of the pancreas with DNA replication errors (RER+) are associated with a characteristic histopathology: poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. Am J Pathol., June 1998
Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal cancer cells. Science 276:1268-1272, 1997.
Zhou W, Sokoll LJ, Bruzek DJ, Zhang L, Velculescu VE, Goldin SB, Hruban RH, Kern SE, Hamilton SR, Chan DW, Vogelstein B, Kinzler KW. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiology, Biomarkers and Prevention 7:109-112, 1998.
Brat DJ, Lillimoe KD, Yeo CJ, Warfield PB, Hruban RH. Progression of pancreatic intraductal lesions (High grade PanIN) to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 22:163-169, 1998.
Griffin CA, Hruban RH, Morsberger LA, Ellingham T, Long PP, Jaffee EM, Hauda KM, Bohlander SK, Yeo CJ. Consistent chromosome abnormalities in adenocarcinoma of the pancreas. Cancer Res 55:2394-2399, 1995.
Hahn SA, Seymour AB, Hoque ATMS, Schutte M, da Costa LT, Reston MS, Caldas C, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. Alleotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res 55:4670-4675, 1995.
Wilentz RE, Chung CH, Strum PDJ, Musler A, Sohn TA, Offerhaus GJA, Yeo CJ, Hruban RH, Slebos RJC. K-ras mutations in duodenal fluid from patients with pancreas cancer. Cancer 82(1):96-103, 1998.
Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res 54:3568-3573, 1994.
Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, Kern SE. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Can Res 54:3025-3033, 1994.

A new type of pancreatic cancer has been discovered by scientists at Johns Hopkins. This cancer was discovered based on a unique type of genetic change preent in the DNA from this cancer.
- April 1, 1998
This discovery was made by identifying a unique type of genetic change present in the DNA of certain pancreatic cancers (M Goggins and colleagues, 1998). Pancreatic cancers with this unique type of genetic change were then analyzed to determine whether they have a distinctive pathology under the microscope. The scientists also examined the survival of people with this form of pancreas cancer after they have had a Whipple operation. Approximately 4% of all adenocarcinomas of the pancreas that were studied had this genetic change, termed microsatellite instability, and these cancers all had a distinctive pathological appearance. Additional genetic changes that are commonly found in the usual type of pancreas cancers such as the K-ras mutation were not present. This suggests that these pancreas cancers arise through a different pathyway than do usual pancreatic cancers.
In some cases, this new type of pancreas cancers can arise as a result of inheriting a defective gene. Most importantly, the group of five people identified with this form of pancreas cancer had a better overall survival. Three of the five patients have survived five or more years after their Whipple operation, and one of the five patients is still alive one and a half years after their operation. Microsatellite instability has been detected in colon and other types of cancers and these cancers have a different response to chemotherapeutic drugs. The same may also be true for pancreatic cancers with microsatellite instability, but further study is needed to answer this question. These are encouraging results and provide hope that by using the powerful technology of genetic analysis to study pancreas cancer, additional discoveries will ultimately lead to further benefits for our patients.
Reference:
Michael Goggins, G. Johan A. Offerhaus, Werner Hilgers, Constance A.Griffin, Manu Shekher, David Tang, Taylor A. Sohn, Charles J. Yeo, Scott E. Kern, Ralph H. Hruban. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology: poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. American Journal of Pathology 1998: 152: 150101507.

Risks of Cancer in Families with Pancreatic Cancer
- March 24, 1998
The National Familial Pancreas Tumor Registry (NFPTR) was established at Johns Hopkins to study families in which there is an aggregation of pancreas cancer, with the hope of learning more about the genetic causes of pancreatic cancer. In the January 1998 issue of The Surgical Oncology Clinics of North America (volume 7, pages 1-23), Drs. Hruban, Petersen, Ha and Kern report the results of their analysis of the families registered to date in the NFPTR. As of November 1, 1997 the registry contained 116 families in which two or more first degree relatives had been diagnosed with pancreatic cancer. These included 38 families in which one generation was affected, 69 families in which 2 generations were affected and 7 families in which three generations were affected by pancreatic cancer. Further analysis of these families revealed that even the second degree relatives (cousins, uncles, etc) of patients with familial pancreatic cancer were at increased risk of developing pancreatic cancer. Remarkably, other cancer types were also increased in the second degree relatives of patients with familial pancreatic cancer. The most common non-pancreatic cancers to be diagnosed in relatives of pancreatic cancer patients included breast cancer, lung cancer, colon cancer, melanoma, bladder cancer and prostate cancer.
These results not only demonstrate that familial aggregation of pancreatic cancer exists, but they also suggest that second degree relatives of familial pancreatic cancer patients are at an increased risk of developing pancreatic cancer and of developing extra-pancreatic cancers. These relatives may therefore benefit from increased cancer screening. Further studies are clearly needed to identify the genes responsible for this aggregation. To learn more about familial pancreatic cancer, click here.
Reference:
Hruban RH, Petersen GM, Ha PK, Kern SE. Genetics of Pancreatic Cancer. From Genes to Families. Surgical Oncology Clinics of North America 7:1-23, 1998.

New Markers for Pancreatic Cancer
- February 18, 1998
In this month's issue of Cancer Epidemiology, Biomarkers and Prevention (Reference #1), Dr. Wei Zhou and colleagues from Johns Hopkins describe the development of a potential new serum marker for pancreatic cancer. The marker, called "tissue inhibitor of metalloproteinase type I (TIMP-1)", had been previously shown to be expressed at high levels in pancreatic cancer using a technique called serial analysis of gene expression. Dr. Zhou and colleagues have now extended this previous study and looked at TIMP-1 levels in the serum of patients with pancreatic cancer. They found that TIMP-1 was increased significantly in the serum of patients with pancreatic cancer, but that TIMP-1, by itself, was inadequate as a serum marker for cancer. However, a combination of TIMP-1, CA19-9 and carcinoembyonic antigen detected 60% of 85 patients with pancreatic cancer in a highly specific manner. This study is exciting, not so much because we believe that TIMP-1 will become a new screening test for pancreatic cancer, but because it demonstrates: (1) that a systematic analysis of gene expression using the SAGE technology can reveal novel serum markers for pancreatic cancer and, (2) because it demonstrates that individually sub-optimal markers can be combined to yield higher sensitivity and specificity for cancer. We believe that further analysis of markers found to be highly expressed in pancreatic cancer using the SAGE technology will lead to the development of novel screening tests for pancreatic cancer.
Reference:
Zhou W, Sokoll LJ, Bruzek DJ, Zhang L, Velculescu VE, Goldin SB, Hruban RH, Kern SE, Hamilton SR, Chan DW, Vogelstein B, Kinzler KW. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiology, Biomarkers and Prevention 7:109-112, 1998.
Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal cancer cells. Science 276:1268-1272, 1997.
1997 ▼
Hopkins Surgeons Report Their Experience With 650 Whipples Performed in the 1990's
- December 18, 1997
Dr. Charles Yeo, a Professor of Surgery at Johns Hopkins with a particular interest in cancer of the pancreas, and colleagues reported their experience with 650 consecutive Whipple resections performed at The Johns Hopkins Hospital in the 1990's in the September issue Annals of Surgery (1).
Between January 1990 and July 1996 650 patients underwent a Whipple resection at The Johns Hopkins Hospital. Dr. Yeo followed these patients to determine the pathology, complications and outcomes. Most of the Whipple resections were performed for cancer of the pancreas (43%), however, Whipples were also performed for ampullary cancers (11%), distal common bile duct cancers (10%), duodenal cancers (4%), chronic pancreatitis (11%), neuroendocrine tumors (5%), periampullary adenomas (3%), cystadenocarcinomas (2%), cystadenomas (4%) and other conditions (7%). The median number of units of red cells transfused was 0 and the median operative time was 7 hours. During this period, 190 consecutive were performed without a mortality. The overall operative mortality rate for this group of 650 patients was only 1.4%. The most common complications included early delayed gastric emptying, pancreatic fistula, and wound infection. The median post-operative length of stay was 13 days.
In multivariate analysis the most powerful independent predictors favoring long term survival included a pathologic diagnosis of duodenal adenocarcinoma, tumor diameter less than 3 cm, negative resection margins, absence of lymph node metastases, well differentiated histology, and no reoperation. This single institution, high volume experience demonstrates that Whipple procedures can be performed safely for a variety of malignant and benign disorders of the pancreas. Overall survival is determined largely by the pathology within the resection specimen.
Reference:
Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Growchow LB, Abrams RA. Six hundred fifty consecutive pancreaticoduodenectomies in the 1900s. Pathology, complications and outcomes. Annals of Surgery 226(3):248-260, 1997.

Hopkins Scientists Develop New Tests to Analyze Liver Lesions Biopsied from Patients with Pancreas Cancer
- October 21, 1997
Patients with cancer of the pancreas often have nodules in their livers. In some patients these nodules are perfectly harmless lesions called "benign bile duct proliferations". In other patients, however, the nodules may be nodules of cancer which have spread from the patients pancreas to the liver. These latter nodules are called "metastatic carcinoma". These different types of nodules can make a big difference in treatment. Surgery to remove a pancreas cancer is effective only in patients in which the disease has not spread beyond the pancreas (called "localized disease"). In contrast, chemotherapy without resection of the primary pancreatic cancer is generally the treatment of choice for patients with liver metastases. Unfortunately, it can be very difficult to distinguish benign bile duct proliferations from well differentiated metastatic pancreatic cancer in the liver. In this month's issue of the American Journal of Pathology research scientists from Johns Hopkins, in collaboration with a group of scientists at the Academic Medical Center in Amsterdam, The Netherlands, report a new gene based test that may help make this distinction.
Most primary pancreatic cancers of the pancreas have mutations in the K-ras oncogene (1). In this study of over 100 liver nodules, the scientists found that when the primary pancreatic cancer had a K-ras mutation, the liver metastases also always had the same mutation. In contrast, the vast majority (94%) of benign bile duct proliferations did not harbor K-ras mutations.
These results suggest that K-ras mutations may be useful in distinguishing benign bile duct proliferations from metastases in the liver, and importantly, they demonstrate a potential clinical application of our improved knowledge of genetics of carcinoma of the pancreas. Other potential applications of tests for K-ras include blood or stool tests for rare pancreatic cancer cells and genetic tests as adjuncts to cytologic diagnoses (1,2).
Reference:
Hruban RH, van Manseld ADM, Offerhaus GJA, van Weering DHJ, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL. K-ras oncogene activation in adenocarcinoma of the human pancreas: a study of 82 carcinomas using a combination of mutatenriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 1993, 143:545-554.
Hruban RH, Sturm PDJ, Slebos RJC, Wilentz RE, Musler AR, Yeo CJ, Sohn TA, van Velthuysen MLF, Offerhaus GJA. Can K-ras codon 12 mutations be used to distinguish benign bile duct proliferations from metastases in the liver? A molecular analysis of 101 liver lesions from 93 patients. Am J Pathol 1997, 151:943-949.
Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res 54:3568-3573, 1994.

Hopkins Researchers Demonstrated the Value of Post-Operative Chemo and Radiation Therapy
- August 8, 1997
Should patients with pancreas cancer get any additional therapy after the tumor has been surgically removed? This important question has finally been answered. In the May 1997 issue of Annals of Surgery (Vol 225, 631-636) Dr. Charles Yeo and his colleagues report the Johns Hopkins experience treating patients with radiation and chemotherapy after they have had a Whipple procedure for pancreas cancer. 174 patients were enrolled in this study and the patients received one of three post-operative treatments. These included: 1) standard therapy [external beam radiation therapy to the pancreatic bed given with two 3-day fluorouracil (5-FU) courses followed by weekly bolus 5-FU for 4 months]; 2) intensive therapy [external beam radiation therapy to the pancreatic bed with prophylactic hepatic radiation given with and followed by infusional 5-FU plus leucovorin for 4 months]; or 3) no therapy (no post-operative radiation therapy or chemotherapy). The Hopkins researchers found that the use of adjuvant chemo radiation therapy was associated with significantly longer patient survival. Patients who received post-operative chemo-radiation therapy had a median survival of 19.5 months compared to 13.5 months without therapy. The intensive therapy group had no survival advantage compared to the standard therapy group. From this study the Hopkins researchers conclude that adjuvant chemo radiation therapy significantly improves survival after Whipple procedure for adenocarcinoma of the head, neck or uncinate process. Indeed, based on these survival data, standard adjuvant chemo radiation therapy appears to be indicated for patients treated by Whipple procedure.
Reference:
Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK, Pitt HA, Lillemoe KD, and Cameron JL. Pancreaticoduodenectomy for pancreatic adenocarcinoma: Postoperative adjuvant chemoradiation improves survival. Annals of Surgery 225(5):621-636, 1997.

A Genetic Fingerprint Proves the Origin of Pancreatic Cancer
- July 1, 1997
The best way to fight pancreatic cancer would be to detect it in its earliest stages. But there has been some uncertainty as to what the earliest form might look like. The pancreases of elderly persons often have ducts with abnormal lining cells, but for years these lesions were largely overlooked as trivial. In the vast majority of cases, the microscopic appearance of these lesions does not readily suggest that they might be worrisome. Indeed, one could calculate that if these ductal lesions were the precursor of pancreatic cancer, then only about 1% of them appear to progress to that stage. These small numbers, and the rather benign microscopic appearance of most duct lesions, have raised interesting questions about the true origin of pancreatic cancer.
In a recent issue of the scientific journal, Cancer Research (June 1, 1997), Drs. Chris Moskaluk, Ralph Hruban, and Scott Kern at Johns Hopkins seem to have finally nailed the culprit. And it is indeed the common, ordinary-looking duct lesion. They used a rare mutation in the p16 cell cycle gene, found in infiltrating pancreas cancer, as a marker to determine if any of the nearby duct lesions were related to the infiltrating cancer. This mutation changed only one of the hundreds of nucleotides (building blocks of DNA) in this gene, and could serve as a genetic "fingerprint" for the origin of the cancer. Remarkably, the same p16 mutation was found in the infiltrating pancreas cancer and in a neighboring duct lesion. Another type of mutation, this time in the K-ras gene, was also shared between the duct and the cancer. The proof was now in: a rather unimpressive duct lesion had, over what was probably the span of many years or decades, evolved into the cancer which finally invaded through the duct wall to become an aggressive tumor. Evidence from other patients was also identified to support this scenario.
A small proportion of duct lesions, about 20%, evolve to become more aggressive-looking, as judged by the microscope. Previous work by the Hopkins investigators and by researchers in Japan (Cancer Research 1994; 54:3568; Cancer Research 1993; 53:953) had indicated that mutations of the K-ras gene, common in pancreatic cancers, also appeared more often in the duct lesions with the most aggressive microscopic appearances. K-ras gene mutations were found predominantly in the ducts having the features of "dysplasia" and "papillary architecture". But a large variety of pancreatic abnormalities harbor mutations of the K-ras gene, and therefore they did not seem to be a very specific fingerprint for the most aggressive lesions. The current work extends this type of analysis by using a more specific marker, the p16 gene. Now that the origin of pancreatic cancer is established, we can work harder to determine how these lesions make the transition to cancer, and which individuals might be at greatest risk for progression of their duct lesions.
Reference:
Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinomas. Cancer Research 57:2140-2143, 1997.

Pancreas Cancer Vaccine Approved For Study
- June 10, 1997
Hopkins clinicians/scientists involved in pancreas cancer research are pleased to announce that the FDA has just approved a new vaccine trial for the treatment of pancreas cancer. We are now enrolling patients into this pancreatic tumor vaccine study. Patients who have newly diagnosed stage I, II, or III adenocarcinoma of the pancreas are eligible. Patients must be able to undergo resection of their pancreatic tumor at The Johns Hopkins Hospital. For more information, please contact one of the following co-investigators:
Pat Sauter, R.N. (410)-955-5718
JoAnn Coleman, R.N. (410)-955-5718
Charles Yeo, M.D. (410)-955-7496
John Cameron, M.D. (410)-955-5166
Keith Lillemoe, M.D. (410)-955-7495
This trial will use an allogeneic irradiated pancreas cancer vaccine generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Such an approach has already proven "feasable, safe and bioactive" in patients with renal cell carcinoma (see Cancer Research vol 57, 1537-1546, 1997).

Gene Expression In Pancreas Cancer
- May 28, 1997
The first step in developing a convenient screening test for a cancer is to identify the proteins that the cancer makes. Once these proteins are identified one can then test blood samples from patients with cancer to see if these proteins are released into and detectable in the blood. For example, prostate cancers make a "prostate specific antigen" which is detectable in the blood and which forms the basis for the current screening test for the detection of early prostate cancers.
In this week's issue of Science Magazine, (Science Vol 276:1268-1272, 1997) Lin Zhang, Wei Zhou, Victor E. Velculescu, Scott E. Kern, Ralph H. Hruban, Stanley R.Hamilton, Bert Vogelstein and Kenneth W. Kinzler from Johns Hopkins report the results of an analysis of a series of pancreas cancers using a new technique called SAGE (serial analysis of gene expression). SAGE is a powerful technique which can be used to identify and quantify all of the genes expressed in a tissue. When the investigators applied SAGE to pancreas cancer they were able to identify a total of 404 transcripts (transcripts are pieces of RNA which code for proteins which might be released into the blood of patients) that were expressed at higher levels in pancreatic cancers as compared to normal. The majority (268) of these transcripts were pancreas-specific; suggesting that this technique could be used to identify new pancreas cancer specific markers which could be used to develop screening tests for pancreas cancer.
Reference:
L. Zhang, W. Zhou, V. E. Velculescu, S.E. Kern, R.H. Hruban, S.R. Hamilton, B. Vogelstein, K.W. Kinzler. Gene Expression Profiles in Normal and Cancer Cells. Science 276:1268-1272, 1997.
V.E. Velculescu, L. Zhang, B. Vogelstein, K.W. Kinzler. Serial Analysis of Gene Expression. Science 270:484-487, 1995.

Hopkins Researchers Show A Genetic Picture of Pancreatic Cancer
- May 1, 1997
For centuries, physicians and the public have wondered about the nature of cancer: What is it? How does it arise? Why do most cancers appear in the later years of life? By the turn of the century, many authorities were already convinced that a cancer represented a highly related aberrant group of cells, the errant offspring of a single original cell of the body. This cell presumably had, through uncontrolled growth and division, given rise to many generations of progeny cells which eventually formed a much larger and ever-proliferating lesion.
By the late 1940s, the mutational theory was gathering considerable excitement, even though the techniques to find mutations in the tumors had not yet been developed. One author in 1949 noted, "The recent work on carcinogenesis and mutagenesis is of ... importance since the rational control of a disease should be based upon its true biological nature" (Yale J Biol Med 21:293, 1949). By the early 1950s, there were attempts to estimate the number of mutations needed. A number of investigators noted that the sharp rise in cancer rates in the elderly could be matched to a mathematical model where the age of a person, when multiplied by itself 4 to 6 times, seemed to match their expected cancer risk. This suggested that nearly half a dozen independent mutations, each with some small chance of occurrence during any one year of life, were needed to form the common cancers (Cancer 4:916, 1951 and Br J Cancer 7:68, 1953, and others). By the late 1980s, there was some success in confirming these estimates, even though the particular mutations and genes involved still were largely unknown.
We now have made great strides in determining what the mutations are and which genes they affect. In the latest issue of the scientific journal Cancer Research (May 1, 1997), Dr. Ester Rozenblum and colleagues report a high-resolution picture of the genetic abnormalities of 40 pancreatic cancers as determined in the Kern Laboratory at Johns Hopkins. Her study probably represents the most precise and exhaustive description of the prevalence of various mutations in a cancer type to date. She finds that most of the cancers involve mutations in at least three to four different genes. Many genes sustained mutations or loss of both of their normal copies, and the number of gene abnormalities was therefore seen to be as high as nine distinct mutations. Each mutation is thought to have aided the evolution of the expanding population of cells into its eventual invasive, cancerous form.
Dr. Rozenblum also found that mutations of any one gene could co-exist with mutations of any other gene she studied. For example, an inherited mutation of the BRCA2 gene was clearly not adequate to cause the pancreatic cancer of one patient. This particular tumor, in order to become fully cancerous, had to develop eight additional mutations in key genes. This is important information, especially for the BRCA2 gene involved in inherited susceptibility to breast, pancreatic, and other tumors. It means that the important tumor-suppressive action of BRCA2 is distinct from the suppressive action of the other genes previously known to involve pancreatic cancer.
When combined with the discovery of the importance of the BRCA2 and DPC4 genes, the past fourteen months have provided discoveries of two totally new avenues for understanding the "true biological nature" of pancreatic cancer. Like the myriad pixels in a digital picture, each mutated gene helps us to construct a clearer picture of this cancer at ever-higher resolution.

Johns Hopkins Awarded Specialized Program of Research Excellence (SPORE) in Gastrointestinal Cancer
- February 12, 1997
The National Institutes of Health have awarded the Johns Hopkins Medical Institutions a Specialized Program of Research Excellence (SPORE) in gastrointestinal cancer. This 5 year award supports a highly interactive multidisciplinary program of transitional research directed at reducing the incidence of and mortality from pancreatic and colorectal cancer. The SPORE includes 4 research programs involving 8 research projects which build upon successful existing research programs. In addition, 4 core laboratories are funded. The programs include:
Project 1 - Translational Application of Molecular Genetics for the Early Detection of Pancreatic and Colorectal Cancer
- Project 1A - "Mutant genes in thesis for early detection of colorectal neoplasms" (Bernard Levin, M.D, MD Anderson)
- Project 1B - "New Genetic Markers for Pancreatic Cancer" (Scott E. Kern, M.D.) This project aims to continue the work of Dr. Kern in identifying new cancer causing genes responsible for the development of pancreatic cancer.
- Project 1C - "Targets for screening in pancreatic neoplasia" (Scott E. Kern, M.D.) The overall goal of this project is to define biologically "high risk" precursor lesions in the pancreas and explore the applicability of gene detection as a clinical screening tool.
Project 2 - "Molecular markers for metastasis in colorectal cancer" (Stanley R. Hamilton, M.D.)
Project 3 - "Prevention of pancreatic and colorectal cancer in genetically destined patients by molecular genetic approaches"
- Project 3A - "Genetic analysis of hereditary colorectal cancer syndromes"(Kenneth Kinzler, M.D.)
- Project 3B - "Agents and mechanisms of tumor prevention in the MIN model of familial adenomatous polyposis" (Stanley R. Hamilton, M.D.)
- Project 3C - "Markers for risk in familial pancreas cancer" (Ralph H. Hruban, M.D.) This project will study inheritance patterns in familial pancreatic cancers. It will take advantage of the database and patients cooperating in the National Familial Pancreatic Tumor Registry . These studies should provide a rational basis for predictive gene testing, and for counseling and screening individuals at risk for the development of pancreatic cancer.
Project 4 - "Novel therapies for pancreatic and colorectal cancer - Identification of T-cell recognition targets on pancreatic adenocarcinoma" (Elizabeth Jaffee, M.D.) . This project will identify tumor antigens expressed by human pancreatic adenocarcinomas. Both CD8+ and CD4+ pancreatic tumor specific T-lymphocyte lines and clones will be generated from lymphocytes isolated from patients with adenocarcinoma of the pancreas following vaccination with a GMCSF secreting pancreatic tumor vaccine. A genetic approach will be used to identify genes encoding for MHC class I restricted antigens. The identified antigens will then be evaluated for recognition by CD4+ T-cell lines and clones. Ultimately, the identification of common pancreatic antigens will allow for the development of generalized gene therapy vaccine approaches that can generate immune responses potent enough to treat adenocarcinoma of the pancreas.
Four cores will support these research programs. These include:
- Core 1 - SPORE administration and communication (Stanley R. Hamilton, M.D.)
- Core 2 - Human tissue resource and logistic core (Stanley R. Hamilton, M.D.)
- Core 3 - Colorectal and pancreatic patient registry (Frank Giardiello, M.D. and Ralph H. Hruban, M.D.)
- Core 4 - Biostatistics (Stephen N. Goodman, M.D., Ph.D.)
Finally, and importantly, the SPORE grant will support a developmental research program headed by Bert Vogelstein, M.D. This program will help fund pilot projects. The SPORE also provides funding for a career development program which will allow for the development of young investigators interested in cancer research.
This SPORE is designed to develop areas of basic science with potential impact on pancreatic and colorectal cancer and to move these promising areas into clinical evaluation including clinical trials. The SPORE is also designed to communicate important findings rapidly into the research community to stimulate investigation, and to bring validated transitional findings into the medical community where the research can ultimately reduce the incidence and mortality of these common cancers.
1996 ▼
Hopkins Scientists Discover that as many as 7% of Patients with Pancreatic Cancer are Born with a Defective Copy of the Breast Cancer Gene
- December 2, 1996
The second breast cancer gene, called BRCA2 was recently discovered by Michael Stratton. As described in the February 15, 1996 "What's New", scientists at Johns Hopkins played a major role in the identification of this gene when they discovered that a small piece of DNA missing from a pancreas cancer was right in the middle of the region in which Dr. Stratton and other scientists were hunting for the BRCA2 gene.
Now that the BRCA2 gene has been discovered and its sequence determined, Hopkins scientists have examined the role that this gene plays in the development of pancreas cancer. Dr. Michael Goggins and colleagues at Johns Hopkins studied 245 patients with pancreas cancer and found that as many as 7% of these patients had a defective copy of the BRCA2 gene. Remarkably, this defective copy was present in the patient's "germline" DNA (normal tissue from the patients). The finding of a germline DNA change in these patients means that these patients were born with a defective copy of the BRCA2 gene and that they inherited this defective copy from one of their parents. This discovery is quite remarkable because it suggests: (1) that there is a link between pancreas and breast cancer; (2) that patients born with defective copies of the breast cancer gene may develop pancreas cancer; and (3) that there is now the ability to selectively test patients for an inherited susceptibility to develop pancreas cancer. Patients found to carry a defective BRAC2 gene can be carefully watched and if they do develop a cancer it could potentially be caught earlier.
While further studies are clearly needed to identify how common the defective copies of the BRCA2 gene are in the general population, and how many patients with defective copies of the breast cancer gene go on to develop pancreas and breast cancer, the findings by Dr. Goggins and his colleagues provide one of the first opportunities for scientists to identify patients who are at an increased risk for pancreas cancer using molecular-based tests.
If you are interested in finding out more about genetic testing for cancer and whether it would be appropriate for you or your family, please contact Karen Johnson, MS, CGC.
Reference:
Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM, Yeo CJ, Jackson CE, Lynch HT, Hruban RH, Kern SE. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res 56:5360-5364.

Hopkins Researchers Help Identify the Structural Basis for Genetic Changes in Pancreatic Cancer
- November 5, 1996
Two approaches can be used to identify the genes which are important in the development of a cancer. "Classical cytogenetics" (a technique similar to the tests performed on fluid obtained by amniocentesis from pregnant women to determine the health of their babies) can be used to visualize and examine individual chromosomes in a cancer. With "molecular techniques", DNA probes specific for each chromosome arm are used to identify specific pieces of DNA that have been lost in a cancer. The lost pieces of DNA are often the sites of mutated genes called "Tumor Suppressor Genes".
Dr. Daniel Brat and his colleagues at The Johns Hopkins University analyzed a series of pancreatic cancers using both of these techniques. They found that most (65%) losses of DNA identified using molecular techniques could also be seen in the classical cytogenetic analyses. Importantly, this study provided Dr. Brat and his colleagues with the unique opportunity to determine the mechanism by which the DNA losses were occurring. For example, Dr. Brat was able to show that 123 of "losses of heterozygosity" (losses of DNA) identified at the molecular level were caused by whole chromosome losses in 83 instances, by partial deletions of a chromosome in 18 cases, by the formation of the structures called isochromosomes in 9 tumors, by the addition of parts of a chromosome in 8 cancers, and by translocation (the movement of a piece of DNA from one chromosome to another) in 5 cancers.
This study advances our understanding of the mechanisms by which genes are lost in pancreatic cancer. Researchers at Johns Hopkins are now expanding this study to look at more pancreatic cancers and to look at specific chromosomes in greater detail.
Reference:
Brat DJ, Hahn SA, Griffin CA, Kern SE, Hruban RH. A comparison of karyotypic abnormalities and allelic loss in surgically resected human pancreatic adenocarcinoma. Presented at the United States and Canadian Academy of Pathology Meetings, 1996. Laboratory Investigation 74:134A:779, 1996.
Brat DJ, Hahn SA, Griffin CA, Kern SE, Hruban RH. The structural basis of molecular genetic deletions: An integration of classical cytogenetic and molecular analyses in pancreatic adenocarcinoma. Am J Pathology (in press), 1996.
Griffin CA, Hruban RH, Long PP, Morsberger LA, Douna-Isssa R, Yeo CJ. Chromosome abnormalities in pancreatic adenocarcinoma. Genes Chrom Cancer 9:93-100, 1994.
Griffin CA, Hruban RH, Morsberger LA, Ellingham T, Long PP, Jaffee EM, Hauda KM, Bohlander SK, Yeo CJ. Consistent chromosome abnormalities in adenocarcinoma of the pancreas. Cancer Res 55:2394-2399, 1995.
Hahn SA, Seymour AB, Hoque ATMS, Schutte M, daCosta LT, Redston MS, Caldas C, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res 55:4670-4675, 1995.

Hopkins Researchers Show DPC4 Gene as Harboring Special Importance for Pancreatic Cancer
- July 1996
Pancreatic cancer has an especially aggressive behavior compared to many other cancer types. This has been a source of frustration for clinicians and researchers, since in many respects pancreatic cancer is very similar to these other tumors. Like most cancers, it is of epithelial origin. It is an "adenocarcinoma", or gland-forming cancer, and thus rather similar to other adenocarcinomas of the breast, colon, rectum, and prostate. Even the known genetic changes of pancreatic cancer often involve the same genes as those found in the other cancer types. But these other tumors can often be cured by surgery, while pancreatic cancer rarely affords a total cure. These considerations suggested that additional clues should be sought, those which distinguished pancreatic cancer from other malignancies.
A recent study suggested one such clue. A gene named DPC4 (for deleted in pancreatic carcinoma, locus 4) was recently isolated by Hopkins researchers. It was found to be mutated in half of pancreatic cancers. To follow up this finding, Mieke Schutte, of Dr. Kern's molecular genetics laboratory, worked in collaboration with investigators of multiple departments at Hopkins and investigators at the NIH National Center for Human Genome Research to investigate the DPC4 gene in other tumor types. She found that, of 338 tumors derived from 12 distinct anatomic sites outside the pancreas, only two had mutations involving the DPC4 gene. When her exhaustive study is combined with some earlier data, we now know that DPC4 is indeed occasionally inactivated in other tumor types, including bladder, breast, ovarian, biliary, and colorectal cancers. But for the other sites studied to date, it appears that DPC4 mutations are distinctly uncommon, involving less than 10% of the tumors occurring outside the digestive tract.
With this study, we begin to show some very clear differences between pancreatic cancer and other common cancer types at the genetic level. It is hoped that through this approach to uncover what is distinctive about pancreatic cancer, we should eventually understand better the special nature of the disease.
Reference:
Schutte M, Hruban RH, Hedrick L, Cho KR, Nadasdy GM, Weinstein CL, Bova GS, Isaacs WB, Cairns P, Nawroz H, Sidransky D, Casero RA Jr, Meltzer PS, Hahn SA, Kern SE. DPC4 gene in various tumor types. Cancer Res 56:2527-2530, 1996.

Hopkins Researchers Help Identify Precursor to Invasive Pancreas Cancer
- April 25, 1996
One of the keys to developing tests to detect cancer of the pancreas earlier is to understand the precursor lesions which give rise to invasive pancreatic cancer. A growing body of evidence suggests that "intraductal lesions" are the precursors to invasive pancreatic cancer. We have previously shown that some intraductal lesions in the pancreas have clonal mutations in codon 12 of the K-ras oncogene, and that many of these intraductal papillary lesions overexpress the p53 gene product (1,2). These findings suggest that these lesions are neoplastic. In addition, we have recently seen a remarkable case that suggests that there is a progression from these intraductal lesions to infiltrating adenocarcinoma of the pancreas. In 1984 a 57 year old man had a distal pancreatectomy and splenectomy at our hospital for irregularities identified within his pancreatic ducts on ERCP. Histologic examination of the resected pancreas revealed chronic pancreatitis and multiple atypical intraductal papillary lesions. The patient did well until 9 years later (1993) when he was diagnosed with an infiltrating adenocarcinoma of the head of the pancreas. This patient's clinical course suggests that intraductal papillary lesions in the pancreas can give rise to infiltrating carcinomas of the pancreas, providing further support for the hypothesis that duct lesions are the precursors of infiltrating adenocarcinoma of the pancreas.
Now John Day, M.D., Joseph DiGiuseppe, M.D., Ph.D. and colleagues at Johns Hopkins have helped identify how these intraductal lesions gain a growth advantage over normal pancreatic ducts (3). They examined the expression of HER-2/neu in pancreata with infiltrating cancers. HER-2/neu expression was examined because upregulation or amplification of HER-2/neu has been associated with a poor prognosis in breast, ovarian, and gastric cancers (4). Nineteen cases were examined and HER-2/neu expression essentially was absent in normal pancreatic ducts and ductules, but, by contrast, HER-2/neu was expressed in 82% of ducts with flat duct lesions, 86% of ducts with papillary duct lesions without atypia, and 92% of ducts with atypical papillary duct lesions and in all specimens with carcinoma in situ. The HER-2/neu proto-oncogene encodes for a 185 kilodalton transmembrane glycoprotein with tyrosine kinase activity. The protein is closely related to the epidermal growth factor (EGF) receptor. When specific ligands, which include EGF and transforming growth factor -alpha, bind to the EGF receptor, the tyrosine kinase activity of the EGF receptor is increased, and the resulting intracellular signals that are generated stimulate cell growth (5). The finding of HER-2/neu overexpression in pancreatic duct lesions suggests that HER-2/neu expression may provide duct lesions with a growth advantage over adjacent non-neoplastic epithelium, thereby promoting the development of adenocarcinoma. Furthermore, it suggests that anti-HER-2/neu monoclonal antibodies or inhibitory ligands that block HER-2/neu could be used to interrupt the proliferative cycle of these intraductal lesions, potentially preventing the development of invasive carcinoma.
Thus, it appears that intraductal lesions are the precursors to invasive pancreatic cancer. These lesions harbor clonal mutations in codon 12 of K-ras and they overexpress the p53 gene product. The demonstration that these duct lesions also overexpress the epidermal growth factor receptor homologue, HER-2/neu, provides further evidence for the hypothesis that lesions formally regarded as various grades of hyperplasia instead may represent neoplasms with the potential for subsequent invasion and metastasis. It is hoped that a better understanding of these early pancreatic neoplasms will lead to the development of tests to detect and treat pancreatic cancers at earlier stages.
Reference:
DiGiuseppe JA, Hruban RH, Offerhaus GJA, et al: Detection of K-ras mutations in mucinous pancreatic duct hyperplasia from a patient with a family history of pancreatic carcinoma. Am J Pathol 144:889-895, 1994.
DiGiuseppe JA, Hruban RH, Goodman SN, et al: Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol 101:684-688, 1994.
Day JD, DiGiuseppe JA, Yeo C, Lai-Goldman M, Anderson SM, Goodman SN, Kern SE, and Hruban RH: Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Human Pathology 27(2):119-124, 1996.
Slamon DJ, Clark GM, Wong SG, et al: Human breast cancer: correlation of relapse and survival with amplification of the erbB-2/neu oncogene. Science 235:177-182, 1987.
Akiyama T, Sudo C, Ogawa H, et al: The product of human c-erbB-2 gene: a 185 kilodalton glycoprotein with tyrosine kinase activity. Science 232:1644-1646, 1986.

Pancreatic Cancer Gene Discovered at Johns Hopkins
- February 27, 1996
In the January 19, 1996 issue of the journal Science, scientists at The Johns Hopkins University School of Medicine announced the discovery of a new pancreas cancer gene (1). Dr. Stephan Hahn, working in the laboratory of Dr. Scott Kern, discovered this gene and named it "DPC4" for Deleted in Pancreas Cancer 4. DPC4 belongs to a group of genes called "tumor suppressor genes". Tumor suppressor genes are those genes that when lost or inactivated contribute to the development of cancer, and DPC4 is mutated or lost in almost half of all pancreas cancers. The new gene is located on chromosome 18, and it may play a vital role in cell function, as it is highly conserved in a variety of species. For example, the DPC4 protein resembles a fruit fly protein called "mad". The gene also appears to function with the transforming growth factor beta (TGFß) family of genes. This family of genes normally inhibits cell growth and one can speculate that loss of the gene would lead to loss of this inhibition and therefore to unrestrained cell growth.
Besides providing a better understanding of the events which give rise to the development of cancer of the pancreas, the discovery of this gene may one day form the basis of a molecular test for cancer of the pancreas. Furthermore, if drugs can be developed to restore its missing suppressor effects, then this gene may provide a new approach to more effective treatments for pancreas cancer (2,3).
The story of the discovery of this gene is the subject of a brief "News and Views" article which appeared in the January 19, 1996 issue of Science (2).
Reference:
Hahn SA, Schutte M, Hoque ATMS, Moskaluk CA, daCosta LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor-suppressor gene at human chromosome 18q21.1. Science 271:350-353, 1996.
O'Brien C. New tumor suppressor found in pancreatic cancer. Science 271:294, 1996.
Hahn SA, Hoque ATMS, Moskaluk CA, daCosta LT, Schutte M, Rozenblum E, Seymour AB, Weinstein CL, Yeo CJ, Hruban RH, Kern SE. Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer Res 56:490-494, 1996.

Hopkins Research in Pancreas Cancer Aids in the Discovery of the Second Breast Cancer Gene
- February 15, 1996
Just as is true for pancreas cancer, breast cancer seems to run in families. Almost 15 months ago researchers discovered the first breast cancer gene, called "BRCA1". This gene causes hereditary breast cancers when it is mutated, but unfortunately it accounts for only half of the cases of hereditary breast cancer. Now, in the December 21/28, 1995 issue of Nature Michael Stratton and his team in the United Kingdom announced the discovery of the second breast cancer gene, called "BRCA2" (1).
It turns out that scientists at Johns Hopkins studying a resected cancer of the pancreas played a crucial role in the discovery of BRCA2. As described in detail in Science (2), Mieke Schutte in the Kern Pancreas Cancer Research Laboratory here at Hopkins found that a small piece of DNA was missing (deleted from) a pancreas cancer. This finding was reported in the journal The Proceedings of the National Academy of Science (3,4). Using the technique of "representational difference analyses" (RDA), Mieke identified a small deletion of chromosome 13q21.2 in a sporadic pancreas cancer. The deletion of less than 200 Kb of DNA involved both copies of chromosome 13, and it was in the region in which other scientists were hunting for the BRCA2 gene. Such deletions often signal the locations of "tumor-suppressor genes", which when lost can lead to cancer. Indeed, the discovery and mapping of this deletion (in a pancreatic cancer!) provided a critical collaboration with Michael Stratton's team, who prioritized this deletion region for the successful gene search for the BRCA2 gene. Indeed, some of the markers previously published by the Hopkins effort included portions of the BRCA2 gene (exon 2 and intron 24). This is an exciting finding, not only because researchers here at Hopkins studying pancreas cancer were able to help in the discovery of the second familial breast cancer gene, but also because there have been some reports suggesting that the risk of pancreas cancer is increased in families with members afflicted with breast cancer (5).
Reference:
Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 378:789-792, 1995.
Marx J. A second breast cancer susceptibility gene is found. Science 271:30-31, 1996.
Schutte M, daCosta LT, Hahn SA, et al. Identification by representational difference analysis of a homozygous deletion in pancreatic carcinoma that lies within the BRCA2 region. Proc Natl Acad Sci USA 92:5950-5954, 1995.
Schutte M, Rozenblum E, Moskaluk CA. An integrated high-resolution physical map of the DPC/BRCA2 region at chromosome 13q12. Cancer Res 55:4570-4574, 1995.
Tulinius H, Olafsdottir GH, Sigvaldason H, et al. Neoplastic diseases in families of breast cancer patients. J Med Genet 31: 618-621, 1994.

Aggressive Surgical Approach for Carcinoma of the Pancreas Improves Survival
- January 8, 1996
The decreases in perioperative morbidity and mortality and improved long-term survival associated with pancreaticoduodenectomy for pancreatic cancer have clearly established a role for this operation when performed with curative attempt. Yet, most surgeons remain hesitant to perform pancreaticoduodenectomy unless surgical margins are widely clear, choosing rather to perform palliative biliary and gastric bypass. This study seeks to determine the role of surgical resection performed as a palliative procedure in patients with small residual carcinoma left at the surgical margins.
A retrospective review from The Johns Hopkins pancreatic carcinoma database was performed which identified sixty-four consecutive patients undergoing pancreaticoduodenectomy for pancreatic carcinoma with gross and/or microscopic evidence of adenocarcinoma at the surgical resection margin. This group of patients was compared retrospectively with sixty-two consecutive patients found to be unresectable at the time of laparotomy due to local invasion without evidence of metastatic disease. Combined gastric and biliary bypass were performed in 87% of these patients. No patients in either group had evidence of either liver metastases or serosal implants. The two groups were similar with respect to age, gender, race and presenting symptoms.
The hospital mortality was identical in both groups. Fifty-eight percent of patients undergoing pancreaticoduodenectomy had an uncomplicated postoperative course, compared to sixty-eight percent of patients undergoing palliative bypass (not significant). The length of hospital stay following pancreaticoduodenectomy was 18.4 days which was significantly longer (p<0.05) than for the patients undergoing palliative bypass (15.0 days). The overall actual survival (Kaplan-Meier) was significantly improved in patients undergoing pancreaticoduodenectomy (p<0.02). Postoperative chemo- or radiation therapy improved survival in both groups.
This study supports the role of an aggressive surgical approach for carcinoma of the pancreas. Pancreaticoduodenectomy can be performed with similar perioperative morbidity and mortality and only a minimal increase in hospital stay when compared to traditional surgical palliation. It would appear that pancreaticoduodenectomy with postoperative chemo- and radiation therapy can be associated with improved long-term survival when compared to patients treated with surgical bypass.
Reference:
Lillemoe KD, Cameron JL, Yeo CJ, Sohn TA, Nakeeb A, Sauter PK, Hruban RH, Abrams RA, Pitt HA. Pancreaticoduodenectomy: does it have a role in the palliation of pancreatic cancer? Ann Surg 1996 Jun;223(6):718-28

